6
PRECAUTIONS
•Read all instructions carefully for the
AtriCure®ASU, Isolator™Transpolar™
Pen, ASU Source Switch, and any
auxiliary device being used prior to
using the devices. Failure to properly
follow instructions may lead to
electrical or thermal injury and may
result in improper functioning of the
device.
•Use of the Pen should be limited to
properly trained and qualified medical
personnel. Proper surgical procedures
and techniques are the responsibility
of the medical professional.
Understanding the proper use of the
Oscor PACE 203 H temporary
pacemaker equipment is also the
responsibility of the medical
professional. Each surgeon must
evaluate the appropriateness of any
procedure based on their own medical
training and experience, and the type
of surgical procedure.
•Patient and procedure selection is
solely a medical responsibility and the
outcome is dependent on many
variables including patient pathology,
and surgical and perfusion
procedures.
•Variations in specific procedures may
occur due to individual physician
techniques and patient anatomy.
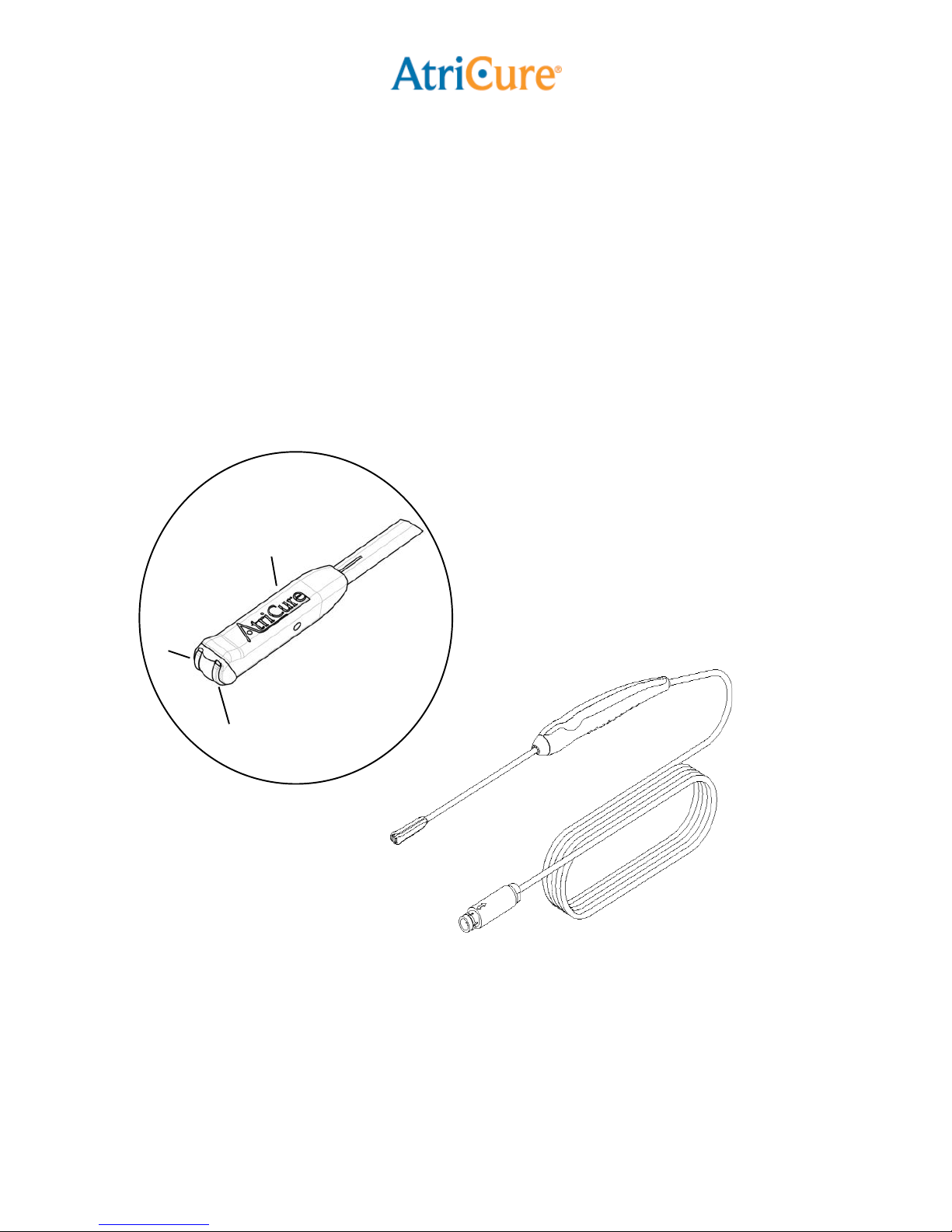
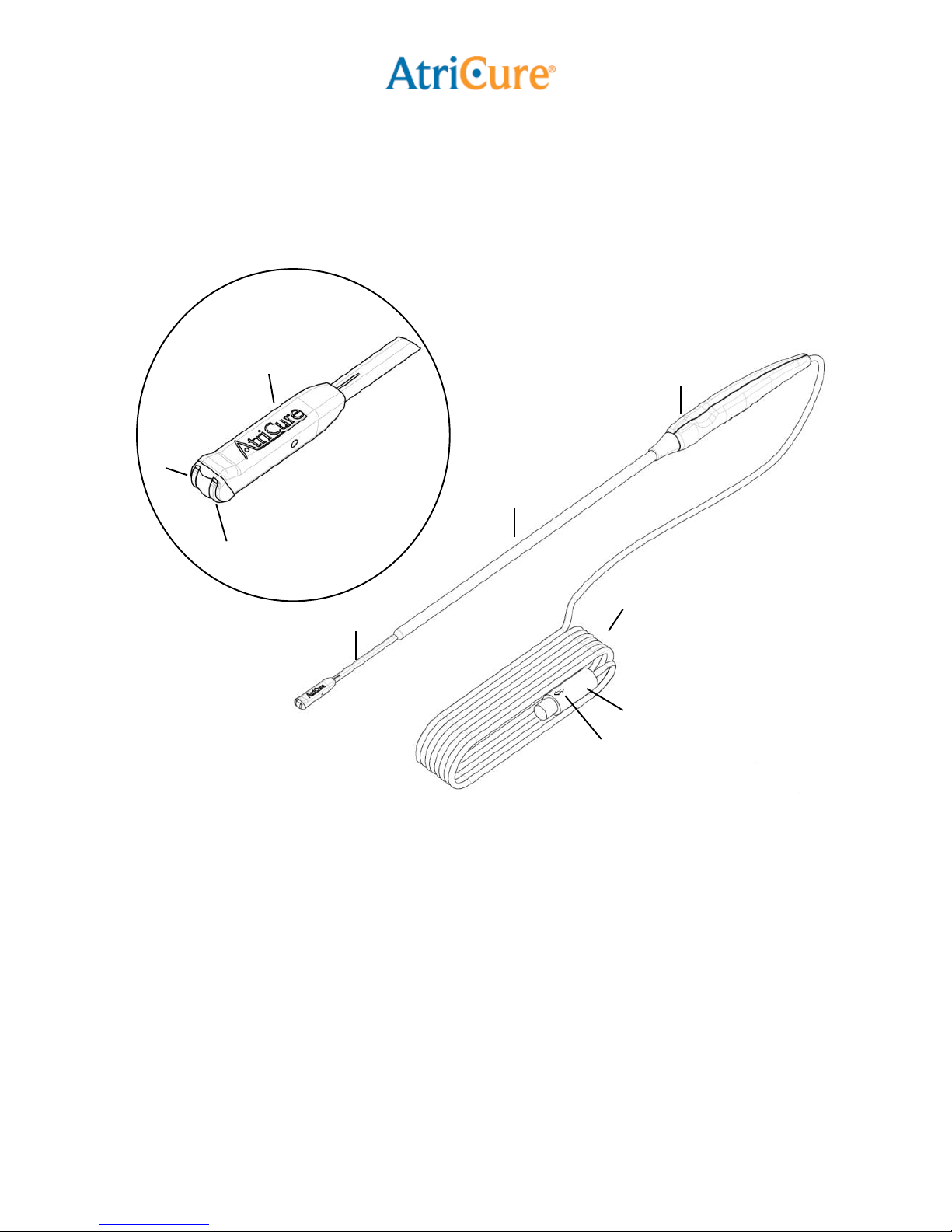
•To avoid damage to the device, do not
drop or toss the Pen. If the Pen is
dropped, do not use. Replace with a
new Pen.
•Do not use the Pen in the presence of
flammable materials.
•Do not re-sterilize or reuse the Pen.
•The distal tip of the Pen must be kept
clean of debris during surgery to avoid
loss of power. Before activating the
ASU, inspect the area at the distal tip
of the Pen for foreign matter. Foreign
matter captured on the tip will
adversely affect the ablation.
•The Pen is only compatible with the
AtriCure®ASU and ASB. Use of the
Pen with another manufacturer’s
generator may damage the device and
result in patient injury.
•The ASB should only be used with
FDA approved cardiac pacing and
sensing devices.
•Do not use the Pen for coagulation or
ablation of veins or arteries.
•Use caution to avoid trauma to tissues
not within the target area of ablation.
Tissue and/or structures behind the
targeted tissue should be protected
from potential thermal spread.
•The Pen has an eight hour useful life
that is tracked by the ASU. If you
attempt to plug in a device that has
reached its time limit expiration, the
Pen will no longer function and the
ASU will display a message indicating
that the Pen must be replaced.
•It is the responsibility of the user to
dispose of this device in accordance
with local regulations.
•Excessive bending of the malleable
stainless steel shaft will cause the
shaft to harden and may increase the
potential for breakage.