©Astute Medical, Inc. 2017 PN 300153 Rev G 2017/01/16
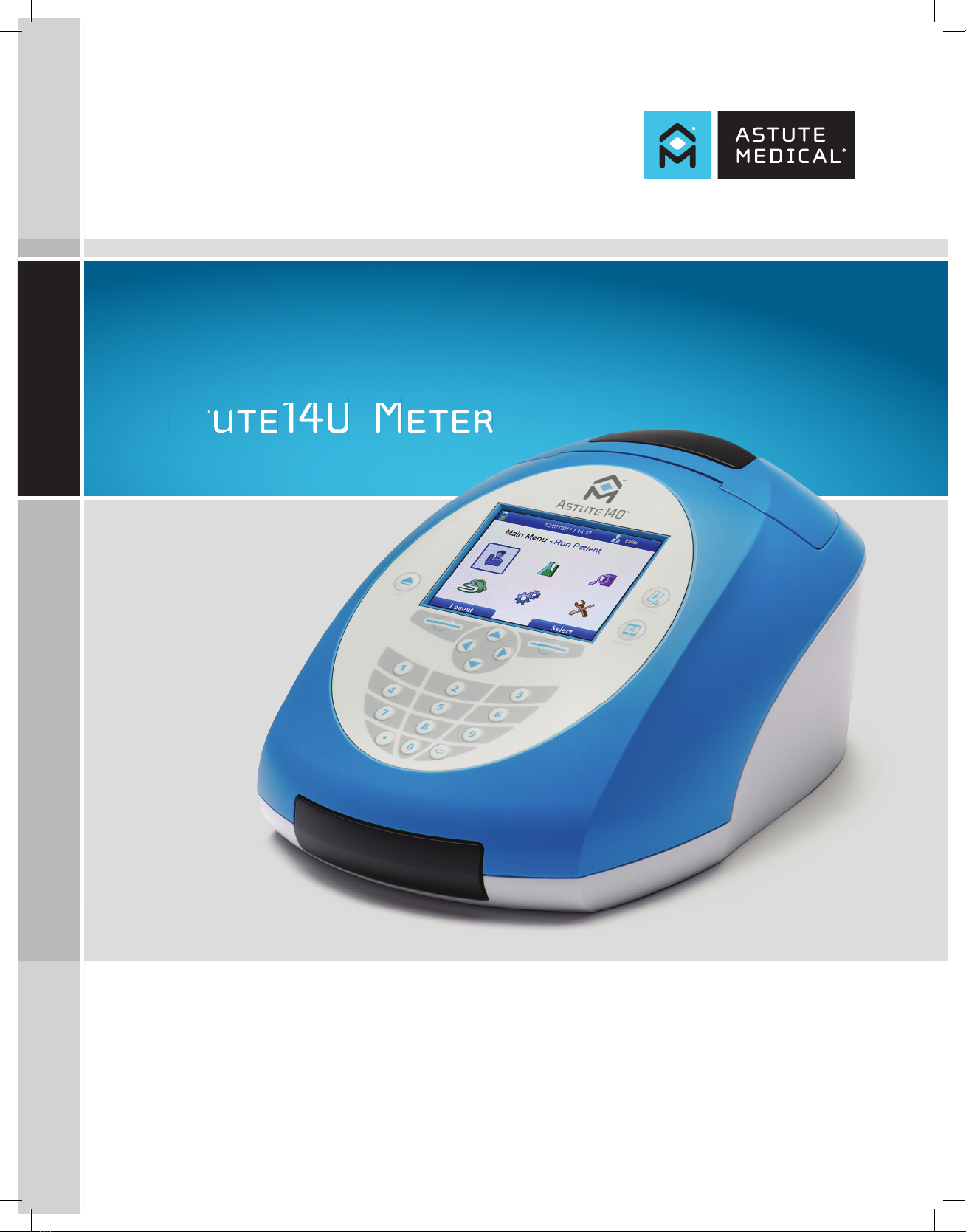
Astute140®Meter
3
Table of Contents
Introduction ............................................................................................................................................... 5
Intended Use ........................................................................................................................................... 5
Principles of Operation ........................................................................................................................ 5
Astute140®Meter Kit Contents ....................................................................................................... 6
Materials Required But Not Provided ............................................................................................ 7
Optional Accessories ........................................................................................................................... 7
Contacting Astute Medical, Inc. (Technical Support) .............................................................. 7
Product Specications ........................................................................................................................ 8
Warnings, Hazards, Precautions and Limitations .................................................................... 9
Safety Symbols ................................................................................................................................ 9
Safety Information .......................................................................................................................... 9
FCC Testing ...................................................................................................................................... 10
Electromagnetic Capability (EMC) .......................................................................................... 10
Limitations ......................................................................................................................................... 11
Astute140®Meter Features .............................................................................................................. 12
User Types .............................................................................................................................................. 15
Operator ............................................................................................................................................. 15
Supervisor ......................................................................................................................................... 16
Installation ................................................................................................................................................ 17
AC Power Supply .................................................................................................................................. 17
Installation and Replacement of Batteries ................................................................................ 18
Installation and Replacement of Paper ....................................................................................... 21
Powering On the Astute140®Meter ............................................................................................ 22
Supervisor Instructions: Conguration and Settings ........................................................... 23
Adding the First Supervisor User ........................................................................................... 23
Setting or Changing Time .......................................................................................................... 26
Setting or Changing Date .......................................................................................................... 27
Setting or Changing the Language ....................................................................................... 28
Updating System Software ....................................................................................................... 29
Updating Astute140®Meter Languages ............................................................................. 29
Operator Registering Permissions ........................................................................................ 29
Quality Control Settings .............................................................................................................. 31
LIS Settings ..................................................................................................................................... 32
Network Settings .......................................................................................................................... 34
PC Mode ........................................................................................................................................... 36
Printer Settings .............................................................................................................................. 37
Managing Users ............................................................................................................................ 38
Astute140®Meter Information ................................................................................................ 40
Error Log ........................................................................................................................................... 42