Augustine Surgical HotDog B1 Series User manual

1
HotDog Patient
Warming Blankets
Models B1XX and B5XX
Instructions for Use
Manufactured by:
EU Authorized Representative:
MDR Importer:
Augustine Temperature Management
15305 Minnetonka Blvd
Minnetonka, MN 55345 USA
TEL: 952.465.3500
FAX: 952.465.3501
www.hotdogwarming.com
Emergo Europe B.V.
Westervoortsedijk 60
6827 AT Arnhem
The Netherlands
NL-AR-000000116
MedEnvoy Global BV
Prinses Margrietplantsoen 33 –Suite 123
2595 AM The Hague
The Netherlands
NL-IM-000000248

Instructions for Use: HotDog Patient Warming Blankets Page 2
INTRODUCTION
HotDog Patient Warming Blankets (“Warming Blankets”) are part of the HotDog Temperature Management
System (System). These instructions apply to the following catalog numbers:
HotDog Product Description
Catalog Number
Qty/Pkg
Warming Blanket, Universal
B500
1
Warming Blanket, Lower Body
B103
1
Warming Blanket, Full Body
B104
1
Warming Blanket, Multi-Position
B105
1
Warming Blanket, Torso
B110
1
A101 cables are available separately.
INDICATIONS FOR USE
The HotDog Temperature Management System is intended to prevent or treat hypothermia and to provide
warmth to patients. The System should be used in circumstances in which patients may not maintain a state of
normothermia. The System can be used with adult and pediatric patients.
The System is intended primarily for use in hospitals and surgical centers including, without limitation,
operating rooms, recovery rooms, emergency rooms, burn units and on other medical/surgical floors.
CONTRAINDICATIONS
•Do not warm ischemic or non-perfused tissue; thermal injury may result. Examples include tissue distal
to aortic cross clamping, or when vasoconstrictive drugs would lead to severe, prolonged vasoconstriction.
•Do not warm patients receiving transdermal medication; increased drug delivery may occur.
WARNINGS
•Explosion Hazard –Do not use the HotDog Controller or Warming Blankets in the presence of flammable
anesthetics or oxygen-enriched environments such as hyperbaric chambers, oxygen tents, etc.
•Do not place Warming Blankets under the patient.
•Inspect Warming Blankets prior to use for signs of damage or excessive wear such as cuts, holes, or loose
electrical connections. If signs of wear are evident, do not use the Warming Blanket until it is inspected by
technical staff.
•Do not continue to use the HotDog Temperature Management System if the over-temperature indicator
and/or alarm continue to sound after reset.
•Do not place Warming Blankets under straps that are used to tightly secure the patient to the OR table.
•HotDog Warming Blankets are not sterile.
•California Proposition 65 Warning: The medical devices and products mentioned in this IFU may contain
chemicals including Urethane or PVC, which is known to the State of California to cause cancer, birth
defects, or other reproductive harm. For more information go to, www.p65warnings.ca.gov
CAUTION
Federal law (USA) restricts this device to sale by or on the order of a licensed healthcare professional.
Page 3 Instructions for Use: HotDogTM Warming Blankets
3
PRECAUTIONS
•Use under the direct supervision of a clinician.
•Monitor the patient’s vital signs regularly during warming according to institutional protocol. If vital sign
instability occurs, notify the clinician.
•Exercise caution when using multiple warming methods.
•Maintain contact between the patient and the labeled sensor on the Warming Blanket.
•Do not fold the Warming Blanket black-side to black-side during use as localized heat build-up may occur
in the overlapped area.
•Always use a barrier (e.g. thin bed sheet, patient gown) between the patient and the Warming Blanket.
•Adjust placement of the Warming Blanket during X-ray imaging as the white labeling and internal wiring
located primarily along the edges of the Warming Blankets may appear in images.
•Do not partially cover the Warming Blanket with thick insulation unless sensor is also covered, or product
damage may occur.
•This device should not be disposed of with general waste at end of life. Follow local regulations for
disposal. The device does not pose any potential hazard.
•Any serious incident that has occurred in relation to this device should be reported to the manufacturer
and the competent authority of the country in which it occurred.
INSTRUCTIONS FOR USE
Follow BEST practices to achieve optimal results, as described in part MT302 BEST Results Poster
(downloadable at hotdogwarming.com). Use only with HotDog controllers.
1. Inspect the Warming Blanket for damage (e.g., cuts, holes, loose electrical connections). Do not use the
Warming Blanket if it is damaged.
2. Place a suitable barrier (e.g. thin bed sheet, patient gown) over the patient.
3. Place the Warming Blanket on top of the barrier, ensuring the heated portion of the Warming Blanket
(black side) faces the patient. On blankets with unheated areas, the heated portions of the blankets are
marked with the “heating area” symbol. (See “Definition of Symbols” section.)
Warning: Do not place the Warming Blanket under the patient and do not roll or fold heated
(black) sides of blankets together during use.
4. Ensure patient is in contact with the Warming Blanket temperature sensor (indicated by a white target).
5. Position and secure the Warming Blanket following the instructions below for the applicable blanket
model. The purple sides of the blanket may be folded back on one another to facilitate effective
positioning.
Blanket Model
Blanket Positioning/Securement Instructions
Lower Body
•The Warming Blanket should maintain its position without securement. If
necessary, tape, Velcro straps, or reusable straps may be used to secure the
blanket as long as it doesn’t create excessive pressure on the patient.
Full Body
Universal
•Secure the Warming Blanket to the patient using the formable edge.
•Connect blankets together using the button and loop connector located on the
sides of the blanket.
Multi-Position
•Secure the Warming Blanket to the patient using the included multi-position
reusable straps, Velcro straps or tape.
Page 4 Instructions for Use: HotDogTM Warming Blankets
4
•Connect blanket segments together using the button and loop connector located
on the inside of the blanket segments. Fit the button and loop together to secure
the two sections.
Torso
•Position the Warming Blanket on the patient, maintaining as much contact as
possible between the blanket and the patient’s chest, arms and shoulders.
•Tuck the unheated insulated flaps around the patient’s neck and shoulders.
6. Insert one end of the A101 cable into the appropriate port on the HotDog Controller.
Controller Model
Port
WC0X
A
WC5X
A or B
WC71
A
WC77
A, B, C or D
7. Insert the other end of the A101 cable into the electrical connector on the Warming Blanket.
8. Turn the HotDog Controller on and select the desired temperature setting to begin warming. The time to
reach the set-point temperature from 23 C +/-2 C is less than 10 minutes. If the blanket does not
reach the selected temperature within 10 minutes, an alarm will sound (Refer to the HotDog Controller
User Manual.)
9. If the HotDog Controller alarm sounds when the Warming Blanket is connected, do not use the Warming
Blanket until the alarm condition is resolved. (Refer to the “Alarms” section.)
10. At the conclusion of warming, remove the barrier and clean the Warming Blanket as necessary. (See
“Care and Maintenance” section below.)
CARE AND MAINTENANCE
•Do not continue to use HotDog Warming Blankets beyond the labeled expiration date, found on the cable.
•Do not launder or sterilize as this may damage the Warming Blanket.
•Do not immerse the Warming Blanket in liquids.
•Do not use high-level disinfectants (e.g. gluteraldehyde, and peracetic acid) or hydrogen peroxide-based
solutions to clean the Warming Blanket.
•Do not spray cleaning solutions into the electrical connector.
•Do not use cleaning or disinfection methods different from those recommended in the Instructions for Use
without first checking with an authorized service representative to ensure that the proposed methods will
not damage the equipment.
•Do not use the Warming Blanket if it shows signs of damage or excessive wear such as cuts, holes, or
loose electrical connectors. Technical staff should perform inspection, such as electrical leakage and
resistance testing, to determine if it is safe for use.
•Do not disassemble the Warming Blanket; the product contains no serviceable parts. If service is required,
call an authorized service representative for assistance.
•Do not fold and crease the Warming Blanket sharply or fold repeatedly in the same location.

Page 5 Instructions for Use: HotDogTM Warming Blankets
5
Storage
Store the Warming Blanket in a dry place, folded or on hanging hooks using the designated holes along the
edges of the blanket. Do not allow the blanket to be cut or crushed. If folding is the preferred method of
storage, please fold loosely and ideally not always in the same manner. Repetitive folding, especially if done
so in a tight manner, may damage internal components and effect the product’s longevity.
Cleaning—General
Clean and disinfect the Warming Blanket between patient uses if it appears visibly soiled. If the Warming
Blanket is not visibly soiled, disinfection at the end of the operating day is recommended. Follow protocols
for non-critical, non-sterile medical devices that may contact intact skin. Examples of similar devices include
blood pressure cuffs, exam table covers, operating room table pads and surgical supports.
Hydrogen peroxide-based cleaning solutions should NOT be used because the vapors degrade the
conductive fabric heaters.
In general, alcohol-based disinfectants are easiest to use since they are fast-acting and can be sprayed or wiped
on the blanket. Other cleaners that are compatible with the outer surfaces of the blanket include sodium
hypochlorite (diluted bleach), phenolic germicidal detergent, and quaternary ammonium detergent. Iodine-
containing cleaners may cause discoloration of the surface material and are, therefore, NOT recommended for
routine cleaning. Dry thoroughly before use.
Caution: Do not place the Warming Blanket in an autoclave, sterilizer, automatic washer-disinfector
or any other high temperature system as this may damage the product.
Cleaning & Disinfection Steps
The cleaning steps below are general recommendations and are not meant to replace hospital-specific cleaning
protocols.
1. Do not allow cleaning fluids to get into the electrical connector.
2. If visible soiling is present, remove before applying a disinfectant. Scrub the affected area with
detergent, using a soft brush or sponge to remove organic matter. Rinse the surface of the blanket
using a dampened cloth. Do not immerse the blanket in liquids.
3. Apply a low- or intermediate-level disinfectant to the entire blanket by spraying or wiping. Follow the
disinfectant manufacturer’s application instructions.
4. Dry thoroughly before use.
ALARMS
All alarm conditions are classified as Medium Priority Technical Alarms. If an alarm occurs, unplug the
device to reset the controller. Check the Warming Blanket and attempt to resolve the alarm. If Alarm Lights
illuminate after a reset is performed, discontinue use and refer the system to Biomedical Engineering. Refer to
HotDog Controller IFU for specific information on the Error Codes displayed.
Page 6 Instructions for Use: HotDogTM Warming Blankets
6
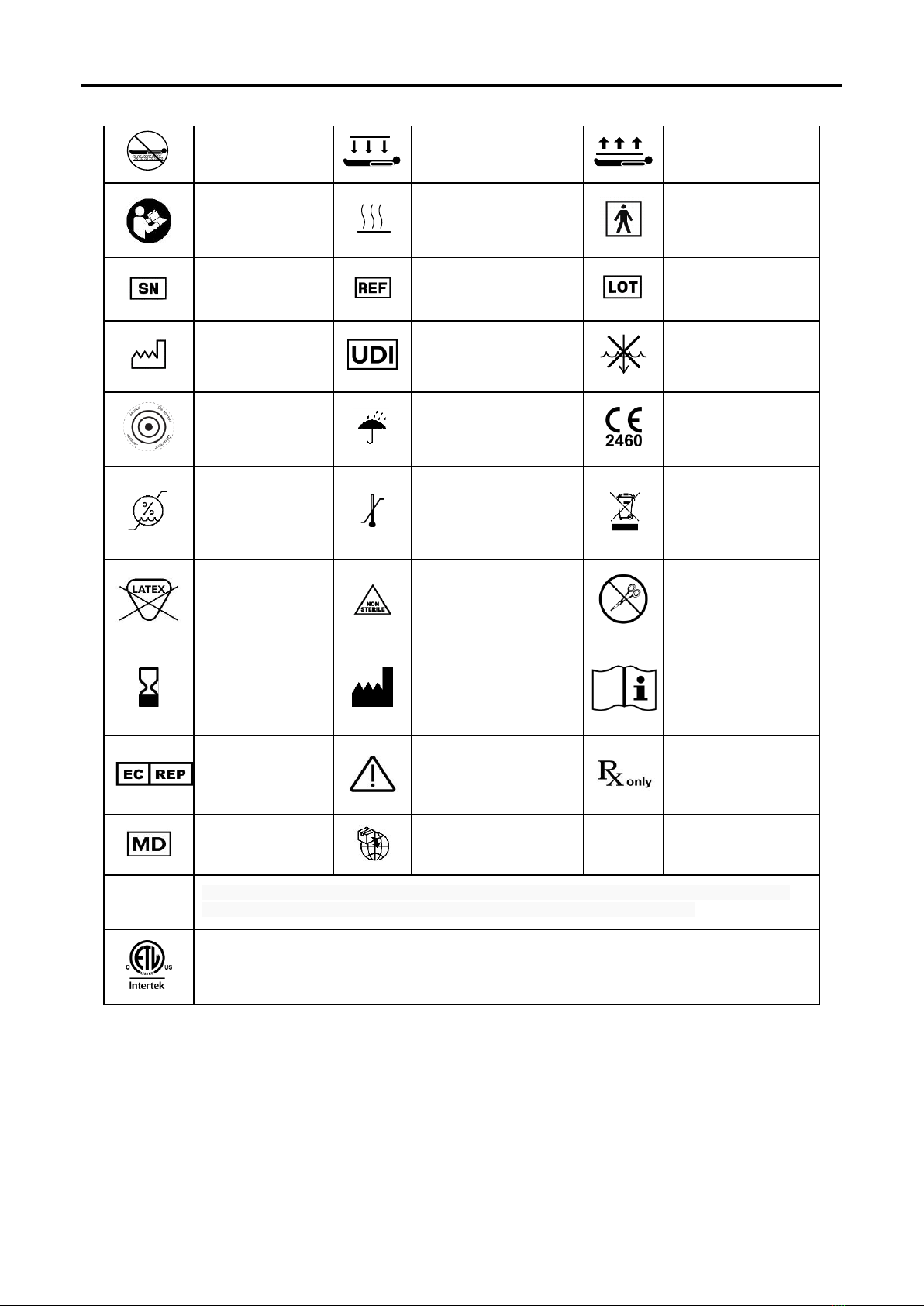
DEFINITION OF SYMBOLS
Do not Place Under
Patient
This Side Down
This Side Up
Attention, consult
accompanying
documents.
Heating Area
BF Patient Applied Part
according to IEC60601-1.
Serial Number
Reference Number
Lot Number
Manufacture Date
Unique Device Identifier
Do not submerge.
Temperature Sensor
Keep Dry
Conforms to European
Medical Device Regulation
2017/745.
Transport and Storage
Humidity Range
Transport and Storage
Temperature Range
Separate treatment from
general waste at end of
life. See Precautions for
details.
Natural Latex Free
Not Sterile
Protect from sharp objects.
Discontinue use if product
is cut or damaged.
Do not use after YYYY-MM-
DD
Manufacturer
Consult the electronic
instructions for use on the
website at the URL
provided.
EU Authorized
Representative
See IFU for Warnings and
Precautions
Medical Device restricted to
sale by or on the order of a
physician
Medical Device
MDR Importer
IPX2
Protected against dripping water when tilted up to 15°; Vertically dripping water shall have no harmful effect
when the enclosure is tilted at an angle up to 15° from its normal position. (The Controller)
Medical Equipment Classified by Intertek Testing Services NA Inc. with respect to electric shock, fire, and mechanical
hazards only, in accordance with UL 60601-1. Classified under the European Medical Device Regulation 2017/745 as a
Class IIb device.
HotDog is a trademark of Augustine Temperature Management, registered in the U.S. Patent & Trademark Office. Devices are
protected by some or all of the following patents: (US Patents 7,543,344; 7,714,255; 7,851,729; 7,786,408; 8,062,343; 8,283,602;
8,604,391; 8,624,164; 8,772,676; 8,986,359; 9,962,122; 9,668,303; 10,154,543; 10,201,935; 10,206,248; 10,506,668; PCT Patent EP
2,062,460) . Other patents are pending.
©2024 Augustine Temperature Management, LLC. All rights reserved. P/N 1718EN Rev K (02/2024)
This manual suits for next models
6
Table of contents
Other Augustine Surgical Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual














