AutoMedx SAVe II+ Series User manual

SAVe II+ Operator’s Manual
INSTRUCTIONS FOR USE

SAVe II+ OPERATOR’S MANUAL > Page 2
Notice to Operators
Please note all indications for use and other applicable contraindications in this
Manual. This manual describes how to operate and maintain the SAVe II+ ventilator.
It is intended to inform responsible parties of the requirements associated with the safe
and effective use of the device.
Federal law (U.S.A) restricts this device to sale by or on the order of a
licensed medical practitioner. Outside the United States check local laws for
any restrictions that may apply.
Read and understand the instructions contained in this manual before
operating the ventilator.
DOCUMENT VERSION
M42110 Rev 5.0 (05/20); Firmware Version R2.0.0
This is the initial release of the SAVe II+ Operator’s Manual. Revisions are expected
to be made to this document as the COVID-19 situation evolves. Please go to
www.automedx.com/support to find current product literature.
The information contained in this manual is applicable to the product with which it was
shipped. Product specifications and features are subject to change without notice.
Always verify the firmware version of this manual (in bold above) matches the
associated device. User environments in which multiple versions of the ventilator are
used must avoid mis-matching manuals.
No part of this document may be reproduced or transmitted in any form or by any
means, electronic or mechanical, for any purpose, without the express written
permission of AutoMedx, LLC. Under the law, reproducing includes translating into
another language or format. The SAVe II+™ logo and the names and marks associated
with AutoMedx’s products are trademarks and/or service marks of AutoMedx, LLC and
are registered and/or common law marks in the United States and various other
countries. Copyright© 2020 All Rights Reserved.

SAVe II+ OPERATOR’S MANUAL > Page 3
TRACKING REQUIREMENTS
U.S. Federal Law (21 CFR 821) requires the tracking of ventilators. Under this law,
owners of this ventilator must notify AutoMedx LLC if this product is:
Received
Lost, stolen or destroyed
Donated, resold, or otherwise distributed to a different organization
If any of the above apply, please visit www.automedx.com/register to register the
device.
AUTOMEDX WILL SEND NOTIFICATION OF SAFETY UPDATES, A RECALL OR
SOFTWARE UPDATES TO THE REGISTERED EMAIL ADDRESS ASSOCIATED
WITH THE DEVICE. THE REGISTERED ORGANIZATION SHOULD UPDATE THE
REGISTRATION WHEN OWNERSHIP IS TRANSFERRED, OR THE PRODUCT IS
DESTROYED.
SOFTWARE/FIRMWARE LICENSE
The software/firmware (“Software”) included in the SAVe II+ product (“Product”) is
owned by AutoMedx and is protected by U.S. and international copyright and other
intellectual property laws and treaties. Your rights to use the Software are subject to
such laws and treaties and the following terms. Your purchase and/or use of the
Product signifies your agreement to these terms.
1) Ownership: All rights to the Software are owned by AutoMedx. The Software is
licensed and not sold. AutoMedx reserves all rights not expressly granted by
these terms.
2) License: AutoMedx grants you a limited, non-exclusive license to use the
Software solely with, and as incorporated into, the Product.
3) Express Limitations: You may not, directly or indirectly, make or distribute
copies of the Software, attempt to discover or gain access to the source code for
the Software, or modify, create derivative works of, disassemble or reverse
engineer the Software.
4) Transfer: You may transfer the Software solely with, and as incorporated into,
the Product, provided that the acquirer of the Product is subject to these terms.

SAVe II+ OPERATOR’S MANUAL > Page 4
Device Warnings
GENERAL
ALTERNATIVE VENTILATION – Always have immediate access to an alternative
means of ventilation, which is ready to use, to reduce the possibility of patient death or
serious deterioration of health.
PATIENT MONITORING – This ventilator is intended to be continuously attended by
an operator. Failure to be in close proximity to this ventilator can contribute to patient
death or serious injury. Such personnel should be prepared to troubleshoot alarms,
address equipment malfunctions and circumstances where equipment experiences
problems.
EXPIRED VOLUME & EXPIRATORY END-TIDAL CO2 MONITORING –This device
is not equipped with expired volume monitoring or CO2 monitoring equipment for
measurement of the expiratory carbon dioxide concentration. The device must be
equipped with suitable expired volume monitoring or CO2 monitoring equipment
(complying with ISO 80601-2-55) before being put into service.
EQUIPMENT COMPATIBILITY - Do not add any attachments or accessories to the
ventilator that are not listed as intended for use in combination with the ventilator in the
instructions for use of the ventilator or accessory, as the ventilator might not function
correctly leading to the risk of patient death or additional serious deterioration of health.
For more information refer to www.automedx.com/accessories
PREVENTATIVE MAINTENANCE - Failure to follow preventative maintenance
procedures could result in device malfunction. For more information refer to
www.automedx.com/service
NOT MRI COMPATIBLE - Do not put the SAVe II+, any components, or accessories
inside an MRI machine.
STORAGE ENVIRONMENT - Storage of the SAVe II+ outside the specified storage
environment may materially impact device performance and permanently damage
and/or shorten the life of the device.
TRANSPORT OF LITHIUM-ION BATTERIES - Regulations govern the transportation
of lithium-ion batteries and devices that have lithium-ion batteries. Check the
appropriate statutes to ensure compliance before transporting the device and / or the
batteries.
UNCERTAIN POWER SOURCES / AUTOMOBILE POWER OUTLETS - Verify the
SAVe II+ internal battery is in good condition and fully charged before connecting the
SAVe II+ AC power supply to uncertain power input sources. Connecting to an
improperly rated power source may damage the AC power supply, preventing the
SAVe II+ battery from charging.

SAVe II+ OPERATOR’S MANUAL > Page 5
PRIOR TO & AFTER EACH DEPLOYMENT
CHARGING BATTERY / EXTERNAL POWER - Only use the battery charger specified
for use with the SAVe II+. The battery should be charged in accordance with the
instructions.
BATTERY - If you suspect the internal battery is damaged, take the unit out of service
immediately. Contact AutoMedx for disposition instruction. DO NOT SHIP DAMAGED
LITHIUM-ION BATTERIES.
RISK OF INFECTION - A patient treated by mechanical ventilation is highly vulnerable
to the risks of infection. Dirty or contaminated equipment is a potential source of
infection. Clean the ventilator and its accessories regularly and systematically before
and after each use and following any maintenance procedure to reduce the risks of
infection.
SAND/DUST/DEBRIS INSIDE MANIFOLD –Do not operate the SAVe II+ if sand,
dust, or other debris have entered the ports.
AUTOCLAVE/STERILIZATION - Never place any part of the SAVe II+ or its
accessories in an autoclave. Unless otherwise indicated, the SAVe II+ and its
accessories are shipped clean, but not sterile.
CONTAMINATED ENVIRONMENT - Take appropriate precautions. The debris filter is
designed to stop particulates, not chemical or biological agents.
IMMEDIATELY PRIOR TO USE
PRE-USE CHECK - Verify functionality of the alarms before connecting the patient to
the ventilator.
CROSS CONTAMINATION - Do not reuse single use accessories. This may cause
cross contamination between patients.
USE OUTSIDE SPECIFIED NORMAL OPERATING CONDITIONS - The performance
of the SAVe II+ may be materially affected if it is used outside of the specified normal
operating conditions.
VENTILATOR PRESETS - Ventilator HEIGHT PRESETS may only be used on adult
patients. Do not use presets when ventilating children. Presets are intended to aid
operators with the initial setup but may not be appropriate for extended periods or in
all situations. Operators should consult their medical director to determine the
suitability of device presets for a given situation.
DURING USE
PATIENT MONITORING –This ventilator is intended to be continuously attended by
an operator. Failure to be in close proximity to this ventilator can contribute to patient
death or serious injury. Such personnel should be prepared to troubleshoot alarms,
address equipment malfunctions and circumstances where equipment experiences
problems.

SAVe II+ OPERATOR’S MANUAL > Page 6
ALARM INDICATORS –DO NOT OBSTRUCT AUDIBLE OR VISUAL ALARM
INDICATORS. Audible indicators may be difficult to hear in noisy environments or if
operator is wearing hearing protection. Do not allow the ventilator’s alarm speaker port
to become covered or obstructed in any way by stickers, labels, clothing, sand, mud,
debris, or other equipment. Take extra precautions to closely monitor the patient and
ventilator in these environments. Verify audible alarm indicators can be heard in the
environment of use.
VISUAL ALARM INDICATORS - DO NOT COVER OR OBSTRUCT VISUAL ALARM
INDICATORSINANYWAY.ALWAYSHAVETHEUSERINTERFACEINVIEW. When
an alarm condition is triggered, or there is evidence of a patient-ventilator fault or
problem, examine the patient first before examining the ventilator.
RESPONSE TO ALARMS –When an alarm condition is triggered, or there isevidence
of a patient-ventilator fault or problem, examine the patient first before examining the
ventilator.
AIRWAY OBSTRUCTIONS - Vomitus and other debris may obstruct the patient end
of the patient breathing circuit. Refer to instructions on clearing debris from the patient
breathing circuit.
SECURE DEVICE - Failure to properly secure the SAVe II+ could damage the device
and could harm the patient by dislodging the breathing circuit or airway. DO NOT
COVER THE VENTILATOR or place in a position that affects proper operation, for
example by placing the ventilator under a blanket or in a position where tension is
placed on the Patient Circuit that may cause it to become dislodged
FIRE HAZARD - Avoid open flames if using supplemental oxygen.
WET ENVIRONMENTS - If using the SAVe II+ in a wet environment take precautions
and protect the device by covering it with a protective barrier.
UNINTENTIONAL CHANGES - To avoid accidental changes to the settings or
inadvertently shutting off the device, verify the user interface is protected from
unintentional contact.
RISK OF EQUIPMENT INTERFERENCE - Potential electromagnetic interference may
occur at levels greater than 20 V/m. Avoid use of the device in environments that may
have high electromagnetic levels. The AC adapter (BATTERY CHARGER,
P/N:M42090) and its associated cables follow the requirements of IEC 60601-1-2.
MAINTAINING AND SERVICING
SERVICE PERSONNEL QUALIFICATIONS - All servicing and repair of the SAVe II+
must be performed by a service technician qualified by AutoMedx. To request a SAVe
II+ SERVICE MANUAL (P/N: M42147) and for qualification requirements visit
www.automedx.com/service
BATTERY REPLACEMENT & DISPOSAL - The SAVe II+ battery should only be
replaced by qualified service personnel. Batteries must be disposed of according to
local environmental legislation. Refer to SAVe II+ SERVICE MANUAL (P/N M42147).
PERSONAL INJURY AND ELECTRICAL SHOCK - DO NOT OPEN THE ENCLOSURE
CASING AND DO NOT USE BATTERIES,AC ADAPTERS,CABLES,OR EXTERNAL POWER SUPPLIES

SAVe II+ OPERATOR’S MANUAL > Page 7
WITH VISIBLE SIGNS OF DAMAGE. Only use power supplies approved by AutoMedx. Refer
to http://automedx.com/accessories for more information.
LIQUIDS –To avoid inadvertent damage, do not pour or spray liquids directly on the
SAVe II+. If liquid cleaners are used, spray on a lint free cloth, then use the cloth to
clean the SAVe II+ and its accessories.
AUTOCLAVE/STERILIZATION - Never place any part of the SAVe II+ or its
accessories in an autoclave. Unless otherwise indicated, the SAVe II+ and its
accessories are shipped clean, but not sterile.

SAVe II+ OPERATOR’S MANUAL > Page 8
TABLE OF CONTENTS
NOTICE TO OPERATORS .............................................................................................2
Document Version.........................................................................................................................................2
Tracking Requirements .................................................................................................................................3
Software/Firmware License...........................................................................................................................3
DEVICE WARNINGS ......................................................................................................4
General..........................................................................................................................................................4
Prior To & After Each Deployment ................................................................................................................5
Immediately Prior to Use ...............................................................................................................................5
During Use.....................................................................................................................................................5
Maintaining And Servicing.............................................................................................................................6
OVERVIEW ...................................................................................................................10
Indications for Use.......................................................................................................................................10
Contraindications.........................................................................................................................................10
Environments of Use ...................................................................................................................................10
Training Requirements ................................................................................................................................11
Risks & Benefits ..........................................................................................................................................11
SAVE II+ VENTILATOR................................................................................................12
Visual Indicators ..........................................................................................................................................12
Front Panel ..................................................................................................................................................13
Alarm Dashboard.........................................................................................................................................15
Back Panel ..................................................................................................................................................16
Port and DC Jack ........................................................................................................................................16
DEVICE ACCESSORIES & ACCOMPANYING DOCUMENTS....................................17
Patient Breathing Circuit..............................................................................................................................18
AC Power Supply / Battery Charger............................................................................................................18
Hard Carrying Case.....................................................................................................................................19
PREPARE FOR USE ....................................................................................................20
Step 1: Unpack Device................................................................................................................................20
Step 2: Verify kit includes the follow required contents:..............................................................................20
Step 3: Verify Patient Circuit .......................................................................................................................20
Step 4: Verify Debris Filter Installation ........................................................................................................20
Step 5: Charge Battery................................................................................................................................21
To charge the battery: .................................................................................................................................21
SETUP FOR USE .........................................................................................................22
Step 1: Establish and Confirm Airway .........................................................................................................22
Step 2: [Optional] Install HMEF into breathing circuit..................................................................................22
Step 3: Connect Patient Breathing Circuit...................................................................................................23
Step 4: Turn on. Select patient height. Press confirm.................................................................................23

SAVe II+ OPERATOR’S MANUAL > Page 9
Step 5: Verify Disconnect Alarm..................................................................................................................24
Step 6: Verify PIP Reached Alarm ..............................................................................................................24
Step 7: Connect Breathing Circuit to Airway ...............................................................................................24
Supplemental Oxygen .................................................................................................................................25
Noise Attenuator..........................................................................................................................................26
REFINING VENTILATOR PARAMETERS ...................................................................28
Respiratory Rate (RR) .................................................................................................................................28
Tidal Volume (TV) .......................................................................................................................................28
RR & TV Combinations ...............................................................................................................................29
Peak Inspiratory Pressure (PIP)..................................................................................................................30
Positive End Expiratory Pressure (PEEP)...................................................................................................30
Displaying Measured PIP and PEEP ..........................................................................................................31
Manually Triggered Breaths ........................................................................................................................31
Manual / CPR Mode ....................................................................................................................................31
SPECIAL PROCEDURES.............................................................................................33
How to Change the HMEF ..........................................................................................................................33
How To Clear Debris or excess fluid From Breathing Circuit......................................................................34
RESPONDING TO ALARMS ........................................................................................35
Alarm Overview ...........................................................................................................................................35
Audible Alarm Indicator ...............................................................................................................................36
DISCONNECT ALARM ...............................................................................................................................37
PIP REACHED ALARM...............................................................................................................................37
BATTERY ALARM ......................................................................................................................................39
DEVICE ALARM..........................................................................................................................................40
HIGH PEEP ALARM ...................................................................................................................................41
LOW PEEP ALARM ....................................................................................................................................41
HIGH MINUTE VENTILATION ....................................................................................................................42
BREATH ALARM.........................................................................................................................................42
BREATH ASSIST ALARM...........................................................................................................................42
MAINTENANCE............................................................................................................43
Battery Maintenance ...................................................................................................................................43
Cleaning ......................................................................................................................................................43
Replace Consumables ................................................................................................................................44
Storage ........................................................................................................................................................45
Scheduled Maintenance ..............................................................................................................................45
APPENDIX A: SPECIFICATIONS ................................................................................46
APPENDIX B: SYMBOLS GLOSSARY........................................................................48
APPENDIX C: RE-ORDER INFORMATION .................................................................51
APPENDIX D: PRINCIPLES OF OPERATION.............................................................52
APPENDIX E: LIMITED WARRANTY ..........................................................................54
APPENDIX F: SOFTWARE RELEASE HISTORY .......................................................55
SAVe II+ OPERATOR’S MANUAL > Page 10
Overview
INDICATIONS FOR USE
The SAVe II+ series are intended to provide ventilatory support for adults during CPR
or when Positive Pressure Ventilation (PPV) is required to manage Acute Respiratory
Failure (ARF) or other situations where mechanical ventilation is needed. The SAVe
II+ series are appropriate for adults that weigh at least 45 kg (99 lbs). It is intended to
be used in pre-hospital, field hospitals, outpatient environments, hospitals, ICU’s,
transport environments or any other healthcare environment requiring the use of a
ventilator.
Federal law restricts this device to sale (or use) on the order of a licensed
practitioner.
CONTRAINDICATIONS
-Should not be used on patients weighing less than 45 kg (99 lbs.)
-Should not be used in situations where Positive Pressure Ventilation (PPV) is
contraindicated
-Do not use device for extended periods without monitoring blood gases. As duration
of use increases, the need for close monitoring of CO2 and O2 levels also increases.
This is especially true for patients over 6’ 9”
-Spontaneously breathing patients may not synchronize with ventilator. If
spontaneously breathing patient has difficulty synchronizing with the device, consider
discontinuing use
-Do not set PEEP above zero (0) when performing CPR
ENVIRONMENTS OF USE
The SAVe II+ is intended to be used in pre-hospital, field hospitals, outpatient
environments, hospitals, ICU’s, transport environments or any other healthcare
environment requiring the use of a ventilator.
Normal Use Environment
The SAVe II+ is intended for use in emergency medical services and healthcare
environment. Performance specifications are based on use in environments with
ambient temperatures of 5 to 45°C (41 to 113°F), relative humidity from 15 to 95%,
and atmospheric pressures from 70 to 110 kPa.
Extreme Operating Environments
Attempting to operate the ventilator outside the temperatures range of -20°C to 50°C
(-4°F to 122°F) may result in ventilator failure and harm to the patient. The extreme
operating environment is when you take the device from a room temperature
environment into an extreme environment and immediately use the device.

SAVe II+ OPERATOR’S MANUAL > Page 11
TRAINING REQUIREMENTS
The device is intended for use by and under the supervision of trained healthcare
professionals, e.g., doctors, nurses, emergency medical technicians, respiratory
therapists and those certified to perform CPR. All operators regardless of experience
or training must be familiar with the contents of this manual and be prepared to provide
primary response to a respiratory emergency. The most up to date information related
to training for operating the SAVe II+ is available at www.automedx.com.
RISKS & BENEFITS
The SAVe II+ is designed to enable providers with limited training to deliver life-
sustaining ventilation to adult patients suffering from Acute Respiratory Failure. The
device is easy to use, lightweight, and intended to be used in any healthcare
environment that requires the use of a ventilator. The operator simply selects the height
of the patient and the device dials in a lung protective Tidal Volume of 6 ml/kg of ideal
body weight, PIP limit of 30 cmH2O and no PEEP. These presets may not be
appropriate for all patients or all conditions. Most ARDS patients will require some
level of PEEP. The operator must continue to monitor the patient and adjust as
necessary.
The SAVe II+ offers a breath-to-breath consistency not achievable with a bag valve
mask (BVM). This is especially important in high stress situations where studies have
demonstrated rescuers are prone to hyperventilating patients. The SAVe II+ delivers
a consistent tidal volume at a consistent rate. In an urgent first-responder situation,
the SAVe II+, unlike a BVM, frees up the responder to address other injuries, attend to
other patients or further assist in transporting the patient. The SAVe II+ at the 5’9”
preset values will provide up to 9.25 hours of ventilation on a full charge. The time
varies depending on settings and patient condition. The SAVe II+ will detect a patient’s
inspiratory effort and automatically trigger a breath.
Unlike pneumatic resuscitators, the SAVe II+ does not require compressed air to
operate, however it will accept low-pressure supplemental oxygen when a higher FIO2
is needed. If in a combat zone, relying on high-pressure oxygen tanks poses a fire and
explosion hazard. These tanks tend to be large and only ventilate for a brief period. If
supplemental oxygen is available and desired, refer to the instructions on page 25.
The operator administering care must monitor the patient to ensure adequate gas
exchange is occurring. The SAVe II+ is designed with multiple system checks to
monitor proper operation of the device and safety of the patient. If an alarm condition
occurs, the SAVe II+ will emit both a visual and audible alarm. In addition, depending
on what triggered the alarm, the SAVe II+ will limit functionality as necessary to avoid
patient injury. For example, the device will trigger an alarm and cutoff power to the
pump when the delivery of additional air exceeds the PEAK INSPIRATORY
PRESSURE (PIP) limit. This safety feature is designed to prevent over inflation and
alerts the medic to fix the fault that triggered the alarm. An alarm troubleshooting label
is attached to the bottom of the device.
The SAVe II+ has adjustable tidal volume (200-800 ml), respiratory rate (8-30 BPM),
peak inspiratory pressure limit (10-60 cmH2O) and positive end expiratory pressure

SAVe II+ OPERATOR’S MANUAL > Page 12
(0-20 cmH2O). However, the I:E ratio is fixed at 1:2 and it does not have an intermittent
mandatory ventilation mode. The max flow rate is 40 LPM and the max minute
ventilation is 12.5 LPM. The device is not intended to be used on patient’s less than
45 kg.
SAVe II+ Ventilator
The AutoMedx SAVe II+ ventilator is a small, extremely durable, portable mechanical
ventilator designed to provide lifesaving mechanical ventilation in prehospital,
aeromedical, field hospital and hospital settings. The SAVe II+ is not a full featured
ICU ventilator, but can play a significant role in extending a hospital’s surge capabilities
during a pandemic or mass casualty situation.
Figure 1: Device Overview
VISUAL INDICATORS
Green LED indicators communicate the current normal operating status of the device.
Green numerical parameter displays communicate device parameter settings and
measured pressures. Like blinking indicators, blinking parameter displays are intended
to signal that operator action is needed to confirm a setting. If the CONFIRM button
(see above) is pressed when all of the parameter displays are solid then measured
pressures (PIP & PEEP) will be displayed for 3 seconds. The CONFIRM button also blinks
for LOW PEEP ALARM, and must be pressed to acknowledge the alarm.
Red alarm codes and the audible alarm indicator signal an alarm condition. Solid
indicators communicate the current device settings or past alarm conditions. Blinking
indicators are intended to signal that operator intervention is needed due to a control
change requiring confirmation or an active alarm condition.

SAVe II+ OPERATOR’S MANUAL > Page 13
FRONT PANEL
Device controls, indicators and displays are located on the front panel of the device
and are organized based on task for rapid setup and troubleshooting.
The device is controlled using membrane buttons. Except for POWER ON/OFF, MUTE
and MANUAL TRIGGER, control changes require confirmation to prevent inadvertent
changes to device settings. Parameter adjustments require operators to select (press)
the appropriate HEIGHT PRESET or the +/- parameter control buttons until the desired
setting is reached then press CONFIRM. The parameter display will blink with the
prospective setting for 10 seconds or until the CONFIRM button is pressed. If not
confirmed, the device will revert to the current device setting and the numerical
parameter displays will turn solid.
Figure 2: Front Panel

SAVe II+ OPERATOR’S MANUAL > Page 14
Table 1: User Interface Overview
REF
Name
Description
1
POWER ON/OFF
Control used to turn device On and Off. Press for 1 second to turn on. Hold for 3
seconds to turn off. The high priority audible alarm indicator will activate 1
second prior to shut down.
2
ADULT HEIGHT
PRESETS
Control and indicator used to set default ventilator parameters based on patient
height and monitor current setting.
3
BATTERY LIFE
Indicates remaining battery life.
4
AUDIBLE ALARM
INDICATOR
Indicates an active alarm condition.
5
EXTERNAL POWER
Indicates external power is connected.
6
ADULT HEIGHT
PRESETS
Indicates device set using preset patient height parameters.
7
USER DEFINED
Indicates device set to user defined parameters.
8
RESPIRATORY RATE
Control and display used to set the RESPIRATORY RATE (RR) and monitor the
set number of breaths delivered each minute
9
TIDAL VOLUME
Control and display used to set the TIDAL VOLUME (TV) and monitor the set
volume in milliliters of gas delivered each breath.
10
PIP
Control and display used to set the PEAK INSPIRATORY PRESSURE (PIP) limit
(pressure cutoff). Once the setting is confirmed the display stays fixed, however,
the device measures the peak pressure breath to breath. To see the last
measured PEAK INSPIRATORY PRESSURE at the Patient Connection Port
press the CONFIRM button.
11
PEEP
Control and display used to set the POSITIVE END-EXPIRATORY PRESSURE
(PEEP) and display the set PEEP of each breath. By pressing the CONFIRM
button during normal operation, the device will display the measured PEEP
maintained in the breathing circuit at the end of exhalation.
12
COMPRESSION RATE
Indicator blinks at a rate of 100/minute to aid users performing chest
compressions when the device is in MANUAL / CPR mode (RR set to zero [0]).
13
MANUAL TRIGGER
Control used to deliver a breath at the set tidal volume.
14
CONFIRM
Control and indicator used to prevent unintended changes. Blinking indicates the
ventilator parameter settings must be confirmed to become active. When all
parameter settings are confirmed (solid) and no changes are pending, pressing
the CONFIRM button will cause the most recent measured PIP and PEEP values
to be displayed in the PIP and PEEP parameter displays for 3 seconds.
15
MUTE
Silences an active audible alarm for 120 seconds. New alarm will override
MUTE. If an alarm condition is still present after 120 seconds the audible alarm
will resume.

SAVe II+ OPERATOR’S MANUAL > Page 15
ALARM DASHBOARD
The alarm dashboard identifies alarm conditions.
Figure 3: Alarm Dashboard
WARNING:
DO NOT BLOCK VIEW OF THE ALARM DASHBOARD. The operator must always have a clear
view of the alarm dashboard when the device is connected to the patient, especially in
noisy environments where caregiver may not hear alarms.
ALARM
DESCRIPTION
DEVICE
The device is outside its temperature range or a software, mechanical or
electrical issue has been detected.
DISCONNECT
The minimum pressure threshold has not been reached during an
inhalation. Most likely caused by a disconnection of the Breathing Circuit
tubing or patient airway.
PIP REACHED
The set Peak Inspiratory Pressure Limit has been reached. Possible
causes include: blockage of Breathing Circuit or airway, low lung
compliance (stiff lungs), excessive tidal volume, and tension
pneumothorax.
BATTERY
Low battery alarm. Audible priority escalates as low battery thresholds
reached.
HIGH PEEP
The measured PEEP is 5 cmH2O above set PEEP. Most likely causes are
blockage of the exhalation port or the patient actively exhaling during the
exhalation phase.
LOW PEEP
The measured PEEP is 5 cmH2O below set PEEP.
HIGH MV
The combination of TV/RR requires a flow rate that exceeds the pumps
ability to deliver at an I:E ratio of 1:2. The device will not permit operator to
select these TV/RR combinations.
BREATH
More than 30 seconds have passed since the last manually triggered
breath. Only active in MASK CPR MODE (RR set to zero).
BREATH ASSIST
Indicates a patient inspiratory effort has been detected and a patient
triggered breath has been delivered.

SAVe II+ OPERATOR’S MANUAL > Page 16
BACK PANEL
The SAVe II+ back panel has two labels. These labels are intended as a reference to
users who have read this manual and the Quick Start Guide. The Basic Setup label
lists the steps to setup the device and includes a table that lists the tidal volumes for
males and females based on height. The Quick Alarm Troubleshooting label lists the
most common conditions associated with each alarm.
PORT AND DC JACK
The SAVe II+ has a label over the ports and next to the DC Jack. These labels are
intended as a reference to users who have read this manual and the Quick Start Guide.
Figure 6: Port Label (Inside Port Cover)
Figure 7: DC Jack Label
Figure 4: Basic Setup
Figure 5: Quick Troubleshooting
SAVe II+ OPERATOR’S MANUAL > Page 17
Device Accessories & Accompanying Documents
The SAVe II+ is kitted with the following accessories and single-use items.
Part Name:
Qty per Kit
Description
Breathing Circuit
(PN: F20066)
1 EA
Channels air to and from the patient’s airway.
Actuates the external control valve and monitors
pressure.
Extendable O2 Reservoir
Tube (PN: F20072)
1 EA
Connects to air intake port and flow regulated
oxygen source. Enables delivery of up to 100%
FIO2 using flow rates up to 12.5 LPM.
Noise Attenuator
(PN: M41112)
1 EA
Mitigates device noise when the oxygen reservoir
or air intake cap is not in use. Fits over intake port.
Debris Filter - Air intake
(PN: F20053)
2 EA (Installed)
Spongey material inside intake port that protects
pump manifold from dust, dirt, and other particles.
Intake Cap - Air Intake
(PN: F20059)
1 EA (Installed)
Black cap that protects the debris filter and intake
port from direct exposure to particles and water.
AC Power Supply
(PN: M42090)
1 EA
Supplies device and battery with external power.
Cord type based on customer location.
Hard Carrying Case
(PN: F20065)
1 EA
Water and dust proof case that protects the system
during transport and storage.
Quick Setup Guide,
SAVe II+ (PN: M42148)
1 EA
Aids the operator’s initial setup by outlining basic
setup instructions and use of the device.
Operator’s Manual,
SAVe II+ (PN: M42110)
1 EA
Instructions for use, storage and maintenance.
Heat and Moisture
Exchanger Filter
(HME or HMEF)
1 EA
(Not in all
kits)
HME provides heat and moisture to the inspired
gas by recycling the heat and moisture contained
in the patient's exhaled gas. An HMEF also filters
viral and bacterial organisms from exhale.
WARNING:
ONLY USE AUTHORIZED ACCESSORIES. Do not add any attachments or accessories to the
ventilator breathing system that are not listed as intended for use in combination with this
ventilator, as the ventilator might not function correctly leading to the risk of patient death
or additional serious deterioration of health. For information on device accessories refer
to www.automedx.com

SAVe II+ OPERATOR’S MANUAL > Page 18
PATIENT BREATHING CIRCUIT
Figure 8: Patient Breathing Circuit Diagram
Oxygen Reservoir Tube
Figure 9: Oxygen Reservoir Tube
AC POWER SUPPLY / BATTERY CHARGER
Figure 10: AC Power Supply / Battery Charger

SAVe II+ OPERATOR’S MANUAL > Page 19
HARD CARRYING CASE
Figure 11: Hard Carrying Case
SAVe II+ OPERATOR’S MANUAL > Page 20
Prepare for Use
To prepare the SAVe II+ for deployment the operator must:
1) Unpack device
2) Verify required contents are packaged in kit
3) Verify Patient Circuit
4) Verify Debris Filter Installation
5) Verify battery has adequate charge and charge as necessary
STEP 1: UNPACK DEVICE
Carefully remove the ventilator and all accessories from the transport container.
Confirm you have received all items listed on the packing slip. Unless otherwise
indicated, the SAVe II+ and its accessories are provided clean, not sterile. It is best to
keep all accessories packaged until needed.
STEP 2: VERIFY KIT INCLUDES THE FOLLOW REQUIRED CONTENTS:
1) SAVe II+, (PN: M50016)
2) Breathing Circuit, (PN: F20066)
3) AC Power Supply, (PN: M42090)
4) Extendable O2 Reservoir Tubing, (PN: F20072)
5) Operator’s Manual, (PN: M42110)
STEP 3: VERIFY PATIENT CIRCUIT
Prior to use, verify the Breathing Circuit (PN: F20066) is new and packaged with
original labeling. Confirm there are no visible signs of damage. Verify shelf life has not
expired.
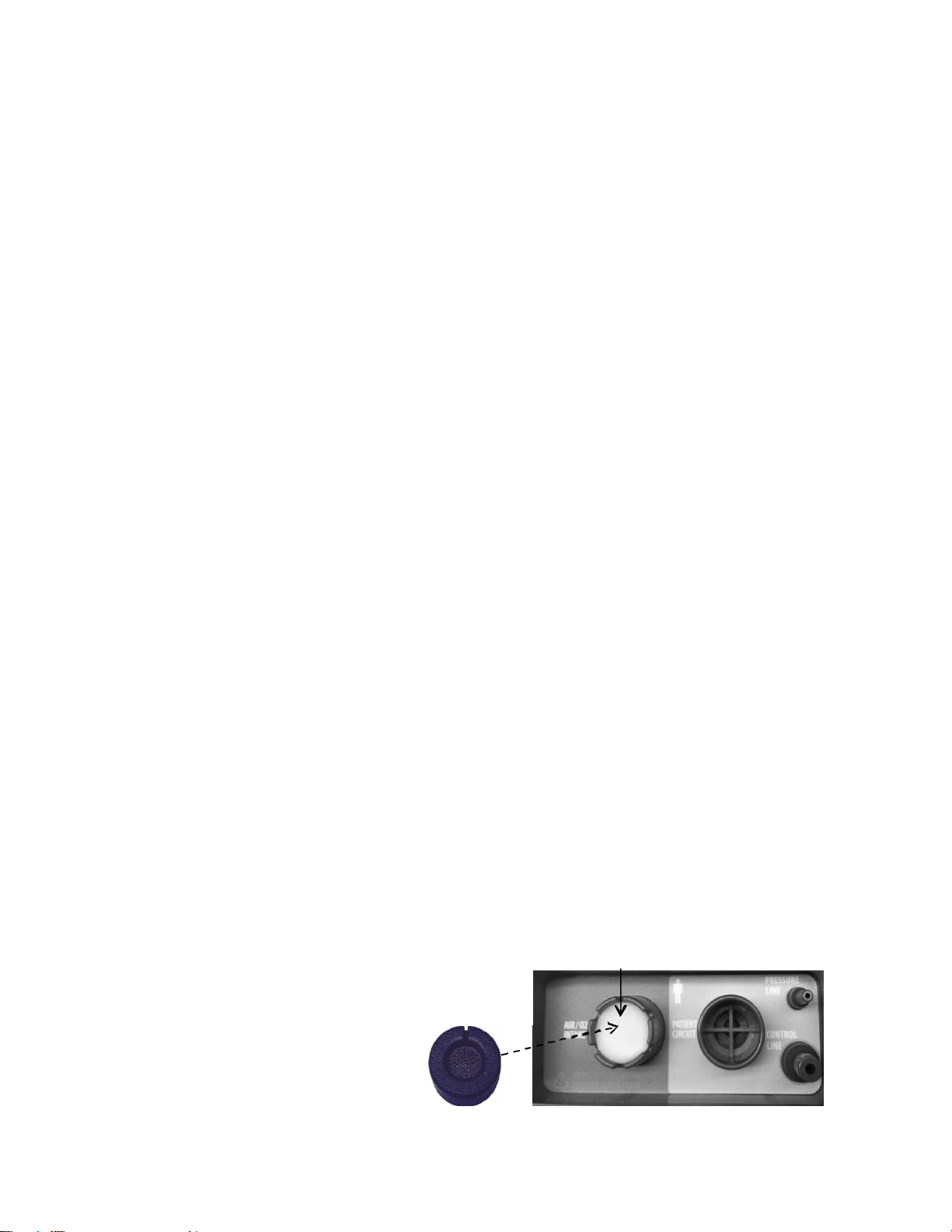
STEP 4: VERIFY DEBRIS FILTER INSTALLATION
The Debris Filter (P/N: F20053) is intended to protect the internal components of the
SAVe II+ system from dust, dirt, and other particles. If using in extremely dusty or dirty
environments two debris filters must always be placed inside the Air/ O2 intake port of
the SAVe II+.
Replace the debris filter after each use if there is any risk of contamination. Inspect the
debris filter prior to each patient use and replace if there is any sign of exposure to
moisture, dust, sand, or other debris.
The Air Intake Cap (PN: F20059)
protects the debris filter from
direct exposure to particles and
water when the attenuator or O2
reservoir is not in use. The Intake Cap
will not obstruct airflow to the patient.
The notches on the intake port permit
sufficient air to be drawn into the pump.
Figure 12: Air Intake Port & Debris Filters
Debris Filter (PN: F20053)
Intake Cap
(PN: F20059)
Table of contents
Other AutoMedx Medical Equipment manuals