AutoMedx SAVe II User manual
SAVe II Operation Manual


SAVe II OPERATION MANUAL Page 3
Preamble
TrademarkInformation
SAVe II™ is a trademark belonging to AutoMedx, Inc. Copyright© 2015 All Rights Reserved.
The SAVe II™ logo and the names and marks associated with AutoMedx’s products are trademarks and/or
service marks of AutoMedx, Inc. and are registered and/or common law marks in the United States and
various other countries. No portion hereof may be reproduced or transmitted in any form or by any means,
for any purpose other than the recipient’s personal use, without the express written permission of
AutoMedx, Inc.
ContactInformation
Manufactured By:
AutoMedx, Inc.
440 Wrangler Dr. Ste 200
Coppell, TX 75019
Phone: (972) 586-7500
Fax: (972) 408-4177
Email: info@AutoMedx.com
Website: www.AutoMedx.com
Authorized Representative in Europe:
(Regulatory Affairs Only)
Emergo Europe
Molenstraat 15
2513 BH The Hague,
The Netherlands
Tel: (31) (0) 70 345-8570
Fax: (31) (0) 70 346-7299
Document Version
M40100 Rev 4.0 (07/16); Firmware Version R1.0.4
If more than 1 year has elapsed since the date above contact AutoMedx to determine if there have been
any updates to the product or to this manual.

SAVe II OPERATION MANUAL Page 4
Notice to Operators
The detailed information and instructions contained within this Operation Manual are designed to ensure
the safe and effective setup, use, and field maintenance of the SAVe II™. The SAVe II must be setup,
operated, maintained and repaired in accordance with the instructions provided in this manual,
accompanying labels and inserts.
It is important that this manual is read and understood in its entirety before operating the ventilator.
Operating or servicing this device without a complete understanding of its characteristics may cause harm
to the patient or user and may permanently damage the device.
The SAVe II is designed for use by trained personnel under the direction of a physician and in accordance
with applicable laws and regulations. This manual describes how to operate and respond to the ventilator,
but does not include instructions on how to respond to the patient. Please contact AutoMedx if the
instructions in this manual conflict with your protocols. Federal law (U.S.A) restricts this device to sale by or
on the order of a licensed medical practitioner. Outside the United States check local laws for any
restrictions that may apply.
Service procedures, including annual calibration verification tests, routine and non-routine maintenance
operations are described separately in the SAVe II™ SERVICE MANUAL (P/N: M40101). For service
information contact: service@AutoMedx.com
FDA Tracking Requirements
U.S. Federal Law (21 CFR 821) requires the tracking of ventilators. Under this law, owners of this ventilator
are required to register the device and to inform AutoMedx if the device is sold or given to another
organization or destroyed. This allows AutoMedx to notify you of safety updates, a recall or software
updates.
Please send the following information to register@automedx.com:
Contact name
Title
Organization name
Street address
City, State, Zip
Contact phone number
Email address
Model Number and Serial Number
Disposition of the device

SAVe II OPERATION MANUAL Page 5
TABLE OF CONTENTS
Preamble........................................................................................................................................3
Notice to Operators ................................................................................................................................ 4
FDA Tracking Requirements .................................................................................................................... 4
Safety Information.................................................................................................................................. 6
General Warning Statements .................................................................................................................. 7
Caution Statements ................................................................................................................................ 8
Use of Symbols ....................................................................................................................................... 9
INTRODUCTION............................................................................................................................10
Device Overview................................................................................................................................... 10
Indications for Use ................................................................................................................................ 11
Contra-Indications ................................................................................................................................ 11
Use Environment .................................................................................................................................. 11
Training Requirements.......................................................................................................................... 11
Features ............................................................................................................................................... 12
Risks & Benefits .................................................................................................................................... 12
DEVICE DESCRIPTION ...................................................................................................................13
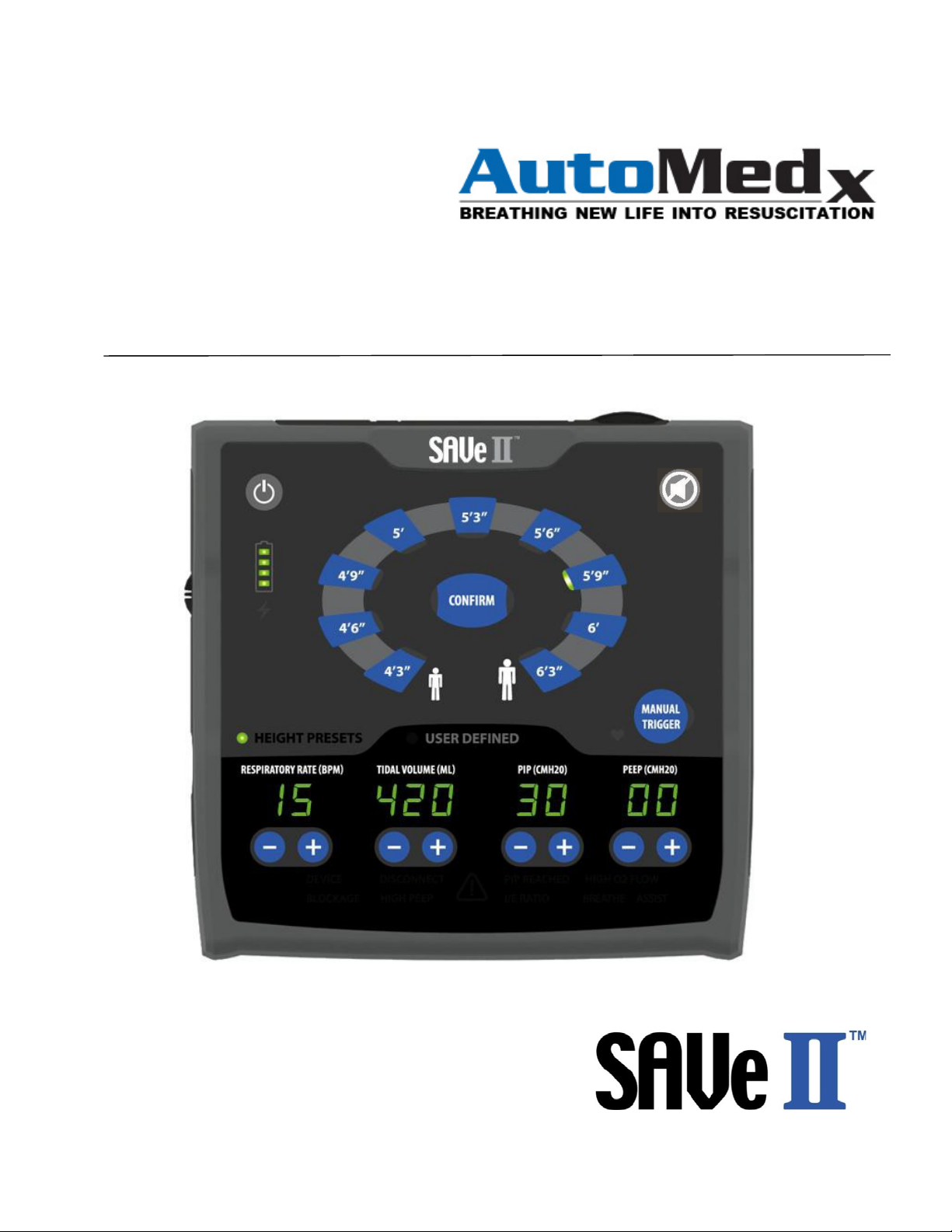
User Interface....................................................................................................................................... 13
Description of Controls, Indicators and Displays..................................................................................... 14
Device Labeling..................................................................................................................................... 15
Alarm Dashboard.................................................................................................................................. 17
Device Disposables & Accessories.......................................................................................................... 18
PREPARE FOR USE ........................................................................................................................19
USING THE SAVE II ........................................................................................................................22
Setup for Use ........................................................................................................................................ 22
Manually Triggered Breaths .................................................................................................................. 31
Clearing Debris from Breathing Circuit................................................................................................... 33
Alarm Overview.................................................................................................................................... 34
Alarm Quick Reference Guide................................................................................................................ 35
RESPONDING TO ALARMS .......................................................................................................................... 37
MAINTENANCE .............................................................................................................................42
REPLACE CONSUMABLES ............................................................................................................................ 44
STORAGE ................................................................................................................................................ 45
SCHEDULED MAINTENANCE ......................................................................................................................... 46
APPENDIX A– SPECIFICATIONS1.............................................................................................................47
APPENDIX B–REGULATORY INFO/CLASSIFICATION/LIMITED WARRANTY .....................................................48
APPENDIX C–PRINCIPLES OF OPERATION .............................................................................................51
APPENDIX D-RE-ORDER INFORMATION .................................................................................................53
APPENDIX E–SOFTWARE RELEASE HISTORY .............................................................................................54
Other manuals for SAVe II
1
Table of contents
Other AutoMedx Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual














