8/24page
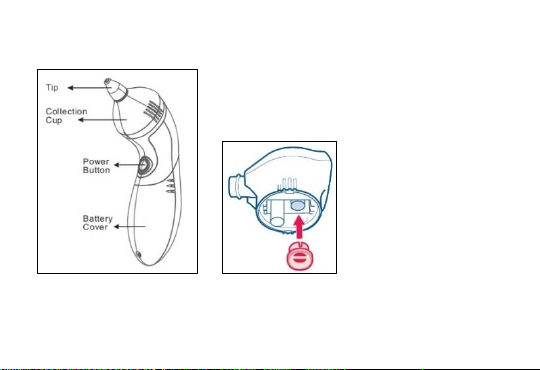
5. Instructions For Use
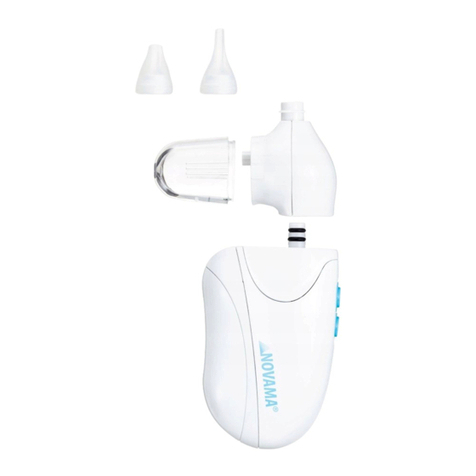
-Choose the correct size included tip based on the anticipated use:
Wider opening tip: recommended for use when the mucus is more
solid.
-Narrow opening tip: recommended for use when the mucus is
more liquid or thin.
-Attached the selected tip firmly to the aspirator unit; it should fit
flush against the unit housing.
-Press the POWER button to activate the suction pressure and
ensure the unit is operating correctly before placing in the nostril.
-Hold the baby or child upright, the aspirator should not be used
while the patient is reclined. Be careful not to place the tip too far
into the nostril or press against the inside wall of the nostril.
-Press the POWER button to turn on the aspirator on. Allow the
suction to clear the mucus. Repeat in the other nostril as needed.
Suction power is mild and should only take a few seconds;
discontinue use immediately if child is uncomfortable.