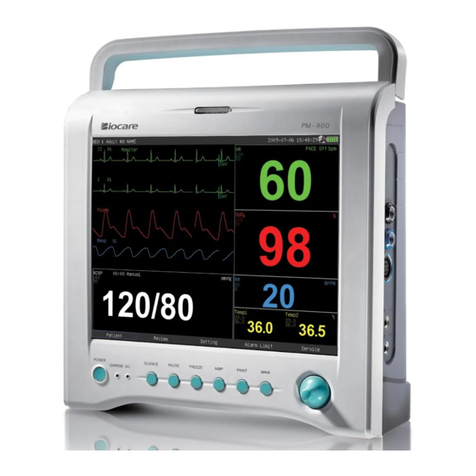
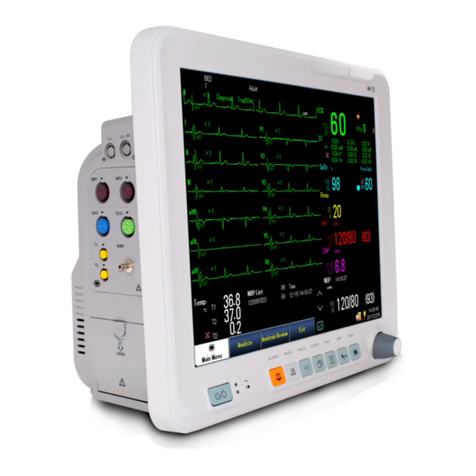
Biocare iM 8 User manual


Version: V1.0 Date: 2018-08
About This User Manual
This manual explains for clinical medical staff how to operate this patient monitor.
Due to continuing product innovation, specifications in this manual are subject to change without notice.
The manufacturer will not be responsible for those indirect or accidental injuries due to misusing the manual or
errors in the content.
The manufacturer reserves the rights to change specifications or stop provide the product without notice. In
addition, the manufacturer will not answer for any sequel because of using the manual.
About Safety
The manufacturer is responsible for the safety, reliability, and performance only if:
Assembly operations, extensions, readjustments, modifications, or repairs are carried out by persons
authorized by the manufacturer or the manufacturer
The electrical installation of the relevant room complies with the requirements of the appropriate government
regulations.
The instrument must be connected to ground correctly and reliably.
The instrument is used in accordance with the instructions for use.

About Service
Contact instrument servicing to the manufacturer or the manufacturer
The manufacturer or their authorized agents will provide telephone, email and other communication services.
In addition to components expanse, payment for service may be required for some kind of services.
Any unauthorized attempt to repair the instrument under warranty voids that warranty.
P must be provided to the manufacturer for services.
Under warranty, Damages due to unartificial factors do not need to take any service expense and components
expense.
Under warranty, Damages due to artificialness only need to take components expense and need not take any
service expense.
Outside warranty, Damages due to artificialness need to take service expense and components expense.
All boards and components that come from repair belong to the manufacture.
Care should be taken in shipping the defective equipment to the manufacturer or the manufacturer
prevent any damage due to shipment.
Shenzhen Biocare guarantees the main unit on the material and the technological qualification for this
product is within 18 months since purchasing day. For accessories and consumable parts, the warranty period
is 6 months. There is no return or exchange in principle.

The CE mark is a protected conformity mark of European
Community. The products herewith comply with the requirements of the
Medical Device Directive 93/42/EEC
Shanghai International Holding Corp. GmbH(Europe)
Eiffestraße 80
20537 Hamburg
GERMANY
Shenzhen Biocare Bio-Medical Equipment Co., Ltd.
#16-1, Jinhui Road, Jinsha Community, Kengzi Sub-District, Pingshan New
District, 518122 Shenzhen, PEOPLE'S REPUBLIC OF CHINA
Tel: 86-755-33005899 Fax: 86-755-27960643
Website: http://www.biocare.com.cn


Contents
iM 8 User Manual -- I --
Contents
About This User Manual..........................................................................................................................................I
About Safety...........................................................................................................................................................I
About Service........................................................................................................................................................II
Contents.....................................................................................................................................................................I
General Warnings for Users ................................................................................................................................... 1
Terms for Safety and Symbols................................................................................................................................ 3
Chapter 1 General Description............................................................................................................................... 5
1.1 Scope ............................................................................................................................................................... 5
1.2 Models and Configurations.............................................................................................................................. 5
1.3 Product Characteristic...................................................................................................................................... 6
1.4 Technical Specifications.................................................................................................................................. 6
1.4.1 Physiological parameters.......................................................................................................................... 6
1.4.2 Power and Interface specifications........................................................................................................... 7
1.4.3 Environment Requirements ...................................................................................................................... 7
1.4.4 Size and Weight........................................................................................................................................ 7
1.4.5 Related instrument.................................................................................................................................... 7
1.5 Conformance Information ............................................................................................................................... 8
1.5.1 EMC.......................................................................................................................................................... 8
1.5.2 Harmonized Standards.............................................................................................................................. 8
Chapter 2 Installation.............................................................................................................................................. 9
2.1 Instrument Installation..................................................................................................................................... 9
2.1.1 Unpacking and Checking.......................................................................................................................... 9
2.1.2 Installation of Support of ward bed .......................................................................................................... 9
2.1.3 Installation of Rollstand............................................................................................................................ 9
2.1.4 Connecting Accessories.......................................................................................................................... 10
2.1.5 Connecting Power Source....................................................................................................................... 10
2.2 Description of the Panel................................................................................................................................. 10
2.2.1 Front Panel.............................................................................................................................................. 10
2.2.2 Side Panel............................................................................................................................................... 12
2.2.3 Rear Panel............................................................................................................................................... 13
Chapter 3 Operation.............................................................................................................................................. 15
3.1 Start to Monitor.............................................................................................................................................. 15
3.1.1 Power On................................................................................................................................................ 15

Contents
-- II -- iM 8 User Manual
3.1.2 Main Monitoring Interface...................................................................................................................... 15
3.2 Patient Login.................................................................................................................................................. 19
3.2.1 New Patient............................................................................................................................................. 20
3.2.2 Continue Patient...................................................................................................................................... 22
3.2.3 Demo ...................................................................................................................................................... 23
3.3 Function Buttons............................................................................................................................................ 23
3.3.1 Function Buttons..................................................................................................................................... 23
3.3.2 Control Knob.......................................................................................................................................... 24
3.3.3 Multi-function key.................................................................................................................................. 24
3.4 Quick Reference Table................................................................................................................................... 25
3.4.1 Setup....................................................................................................................................................... 25
3.4.2 Patient..................................................................................................................................................... 28
3.4.3 Alarm...................................................................................................................................................... 29
3.5 System Parameter Defaults............................................................................................................................ 30
Chapter 4 Working Status Setup.......................................................................................................................... 31
4.1 System Parameters Setup............................................................................................................................... 31
4.2 ECG Parameters Setup (Optional function)................................................................................................... 32
4.3 NIBP Parameters Setup ................................................................................................................................. 34
4.4 SPO2Parameters Setup.................................................................................................................................. 35
4.5 EtCO2Parameters Setup (Optional) .............................................................................................................. 36
4.6 Other Parameters Setup ................................................................................................................................. 37
Chapter 5 Alarm.................................................................................................................................................... 39
5.1 Alarm Parameters Setup ................................................................................................................................ 39
5.2 Alarm Indicators ............................................................................................................................................ 41
5.3 Alarm Control................................................................................................................................................ 42
5.4 Alarm record and history............................................................................................................................... 44
Chapter 6 Patient Monitoring .............................................................................................................................. 45
6.1 ECG and RESP Monitoring (Optional) ......................................................................................................... 45
6.1.1 Preparation.............................................................................................................................................. 45
6.1.2 Display interface..................................................................................................................................... 48
6.1.3 Setup menu............................................................................................................................................. 49
6.1.4 RESP Monitoring (Optional).................................................................................................................. 51
6.2 NIBP Monitoring (Standard) ......................................................................................................................... 51
6.2.1 Preparation.............................................................................................................................................. 51
6.2.2 Display interface..................................................................................................................................... 56
6.2.3 Setup menu............................................................................................................................................. 56
6.2.4 Calibration.............................................................................................................................................. 58
6.3 SPO2Monitoring (Standard).......................................................................................................................... 58

Contents
iM 8 User Manual -- III --
6.3.1 Preparation.............................................................................................................................................. 58
6.3.2 Display interface..................................................................................................................................... 68
6.3.3 Setup menu............................................................................................................................................. 69
6.4 TEMP Monitoring (Standard)........................................................................................................................ 70
6.4.1 Preparation.............................................................................................................................................. 70
6.4.2 Display interface..................................................................................................................................... 74
6.4.3 Setup menu............................................................................................................................................. 74
6.5 EtCO2Monitoring (Optional)........................................................................................................................ 76
6.5.1 Preparation.............................................................................................................................................. 77
6.5.2 Display interface..................................................................................................................................... 81
6.5.3 Parameters Setup .................................................................................................................................... 82
6.5.4 Zeroing Procedure .................................................................................................................................. 83
6.5.5 Alarms..................................................................................................................................................... 84
Chapter 7 Reviewing and Printing....................................................................................................................... 87
7.1 Reviewing...................................................................................................................................................... 87
7.1.1 NIBP Table.............................................................................................................................................. 87
7.1.2 TREND Table ......................................................................................................................................... 87
7.1.3 TREND Graph........................................................................................................................................ 88
7.1.4 Trend Ruler............................................................................................................................................. 89
7.2 Printing.......................................................................................................................................................... 89
7.2.1 Installation of Recorder .......................................................................................................................... 89
7.2.2 Installation of Printing Paper.................................................................................................................. 90
7.2.3 Printing Setup......................................................................................................................................... 91
7.2.4 Printing Report........................................................................................................................................ 92
Chapter 8 Maintenance......................................................................................................................................... 93
8.1 Clean and Sterilize......................................................................................................................................... 93
8.1.1 Exterior Surface...................................................................................................................................... 93
8.1.2 Display.................................................................................................................................................... 94
8.1.3 Cleaning applied parts ............................................................................................................................ 94
8.1.4 Other....................................................................................................................................................... 97
8.2 Replace Power Fuse....................................................................................................................................... 97
8.3 Charge up the Internal Battery....................................................................................................................... 97
8.4 Install and Replace the Battery...................................................................................................................... 97
8.5 Periodic Check............................................................................................................................................... 98
8.6 Service........................................................................................................................................................... 99
8.7 Transportation and Storage............................................................................................................................ 99
Chapter 9 Trouble Shooting................................................................................................................................ 101
9.1 Simple and Apparent Malfunction Checking............................................................................................... 101

Contents
-- IV -- iM 8 User Manual
9.2 Malfunction Instructions Displayed on the Screen...................................................................................... 102
9.3 Error Code Displayed on the Screen............................................................................................................ 103
9.4 Other Phenomena of Malfunctions.............................................................................................................. 104
AppendixA Product Specifications.................................................................................................................... 107
A.1 Safety Information...................................................................................................................................... 107
A.2 Environment Requirements ........................................................................................................................ 107
A.3 Power Supply Requirements....................................................................................................................... 108
A.4 ECG (Optional)........................................................................................................................................... 108
A.5 NIBP........................................................................................................................................................... 109
A.6 SPO2.............................................................................................................................................................111
A.7 RESP (Optional)..........................................................................................................................................112
A.8 TEMP...........................................................................................................................................................113
A.9 EtCO2(Optional).........................................................................................................................................113
Appendix B EMC Statement................................................................................................................................117
B.1 Electromagnetic emissions...........................................................................................................................117
B.2 Electromagnetic immunity...........................................................................................................................118

General Warnings for Users
iM 8 User Manual -- 1 --
General Warnings for Users
Caution
Federal law restricts this device to sale by or on the order of a physician.
Warning
All Users must read following warnings, cautions and guide before operating the Monitors. Our company will not
be held responsible nor any warranties will be made by us for any abnormalities or malfunction of the monitor or
body injury caused by the violations of the operational guides.
General Warnings
The instrument is not a therapeutic instrument.
This instrument must be operated under the direction of professional medical staff.
All of the monitoring parameters are used as a reference and should not be used as the clinical diagnosis. For
abnormalities, clinical methods should be used to check out the reasons.
The instrument should not be operated in the circumstance with flammable gas or corrosive gas.
Prevent ingress of liquid or electrical conductive substance into the instrument.
The instrument must be grounded correctly, and the power supply must be in accordance with the specified
requirement.
Delete all the previous data when monitoring a new patient. Only one patient should be monitored at once.
If monitor connects to the other instrument, the leakage current must be tested by qualified technician before
use, and must comply with IEC 60601-1.
Many components may be attached to this monitor, but the entire unit, with accessories must comply with
IEC 60601-1.
The connection must present no danger to the patient. Check cabling prior to attaching to the patient.
FDAapproved ECG defibrillation proof cables must be used with this monitor.
Checkout the alarm system periodically.
Do not touch the patient in defibrillation. Otherwise, it may lead to serious injury or death.
A
When using with a pacemaker or other electric equipment, no parts can be connected with patients, except
FDAapproved defibrillation ECG cable.
In order to avoid of burning the patients, high frequency electrical bistoury cannot touch the electrode when
used with the monitor.
Do not place the electrode onto the injured or edematous site to prevent infection.

General Warnings for Users
-- 2 -- iM 8 User Manual
Do not measure the blood pressure on the limbs with catheter or infusion. Do not put on the cuff at or near
the wounded position.
Local bleeding may be caused when using the blood pressure monitoring in patients with severe bleeding
tendency. Be careful when using on patients with sickle cell disease.
Do not place the SPO2sensor onto the injured skin, edematous or fragile tissues.
Discomfort or pain may be caused by the continuous use of the clip type SPO2sensor especially in patients
with microcirculation disorder. Do not place the sensor over 2 hours at the same place.
EtCO2cannot be used as the only means of monitoring a patient. It shall always be used in combination with
other vital signs monitoring devices and/or professional human judgments of patient condition.
Non-disposable accessories should be sterilized before used on next patient to prevent cross infection.
The instrument can only be opened or repaired by authorized personnel by manufacturer.
Users may not be notified for changes of accessories.
Please deal with the package waste according to the local regulations.
Warnings and Notes Especially for Neonates
1. ECG Measurement
Warning: Keep ECG away from the throats of the neonate to avoid asphyxiation.
Caution:
tender. Check timely and change the electrodes when necessary.
2. NIBP Measurement
Warning:
pressure of the cuff can cause injury to the neonates.
Caution: Select a cuff that fit the size of the neonate before the neonate measurement. Properly set the
attention to the neonate parameters if you have to adjust parameters.
3. SPO2Measurement
Caution: SPO2may be not obtained precisely because the neonate moves. To measure precisely, please keep
the neonate at rest.
Caution: Use the proper probe to neonate. Do not place the SPO2probe on the fingers that have skin injury,
edema or fragile. Do not place the probe on the same finger over 2 hours to prevent discomfort of the finger.
Check timely and change the fingers when necessary.
4. TEMPMeasurement
Warning:
5. EtCO2Measurement
Warning: Avoid direct contact between the EtCO2probe and the infant's body, or an insulation material must
be placed between the probe and the body.

Terms for Safety and Symbols
iM 8 User Manual -- 3 --
Terms for Safety and Symbols
Terms for Safety
In this manual, Warning, Caution, and Note are used to describe the level of danger. Please be familiar with
their definition and meaning.
Warning: Instructions to avoid potential danger and incorrect operation. Obey the instructions, otherwise death
and serious injury may be caused.
Caution: Instructions to avoid potential danger and incorrect operation. Obey the instructions, otherwise injury,
equipment failure or data loss may be caused.
Note: Operational instructions or other useful information to help the users to operate the instrument
correctly.
Symbols
The followings list of symbols may be used in this instrument.
This symbol
CE mark that complies 93/42/EEC as amended by 2007/47/EC medical device directive. It shall
be accompanied by the identification number of the notified body beside the right bottom side of
CE mark.
Followed by the serial number of theinstrument.
Followed by the name and address of the authorized representative in the European Community.
user
Defibrillation-proof type CF applied part, on medical equipment to identify a defibrillation-proof
type CF applied part.
Equipotentiality, to identify the terminals which, when connected together, bring the various
parts of an equipment or of a system to the same potential. Not necessarily being the earth
(ground) potential. e.g. for local bonding.
The symbol indicating separate collection for electrical and electronic equipment.
Indicating network interface.

Terms for Safety and Symbols
-- 4 -- iM 8 User Manual
-- Blank Page --

General Description
iM 8 User Manual -- 5 --
Chapter 1 General Description
Note:
This manual contains information about this Patient Monitor.
All operators must read and understand this manual before using the monitor.
All information in this manual, including the illustrations, is based on a monitor with all optional
configurations. If your monitor lacks any of these options, some information in this manual may not apply.
Caution:
Federal law restricts this device to sale by or on the order of a physician.
1.1 Scope
This instrument is a battery or line-powered patient monitor. The patient monitor acquires the physiological
signals such as Electrocardiograph (ECG), Respiration (RESP), Non-Invasive Blood Pressure (NIBP), Pulse
Oximetry (SPO2) and End-Tidal CO2(EtCO2). The signals are converted into digital data and processed, examines
the data for alarm conditions and displays the data. The monitor also provides operating control for the user.
The patient monitor is intended to be used in a hospital clinical area such as intensive care units, cardiac care units,
operation room, and emergency department, to provide additional information to the medical and nursing staff
about the physiological condition of the patient.
1.2 Models and Configurations
This instrument can be configured with the following physiological parameter monitoring modules: ECG/RESP,
NIBP, SPO2, TEMP and ETCO2. To meet with different application needs, some different combinations of these
modules can be selected. The following table shows the selectable model configurations:
Item
Configurations
1
NIBP, SpO2
2
NIBP, SpO2, TEMP
General Description
-- 6 -- iM 8 User Manual
1.3 Product Characteristic
Portable structure;
LCD+LED display, high resolution, no distortion, high brightness, great view angle LCD;
Multi-parameter and all-round assistant function;
Modularized design, and easy for maintenance;
Monitoring multi-parameters simultaneously, alarm signals will be raised when a parameter overlaps its
limit;
Waves and parameters will be saved when there is an alarm, 3500 groups of alarm data can be saved at most;
All alarm records can be reviewed afterwards;
No increased leakage current when some instruments connected together;
Powered with AC power, internal battery or vehicle DC power supply, the optional high capacity internal
Li-ion battery can support 10 hours of normal working;.
Drop proof design, helpful for first aid medical services;
Waveforms can be freezed and saved, and SD card can be used for data storage;
Several different types of temperature probes can be selected.
1.4 Technical Specifications
1.4.1 Physiological parameters
The instrument can be configured with several function modules which supports the monitoring of the following
physiological parameters:
Physiological Parameters
ECG
Electro-Cardio Graph, 3/5 leads selectable
NIBP
Non-Invasive Blood Pressure, Oscillometric method, SYS/DIA/MAP.
The manufacturer or SunTech NIBP module is selectable.
SPO2
Saturation of Pulse Oxygen, Golden standard module supported.
The manufacturer or Nellcor or Masimo SPO2module is selectable.
RESP
Respiration, thorax impedance method with ECG
TEMP
Temperature, Fast detect module selectable.
Conventional temperature probe or infrared Thermometer probe is selectable
EtCO2
End-Tidal CO2, Mainstream and sidestream method.
Some of the function modules have several options to meet the special requirements of user.
For detailed parameters, please refer to appendixA.

General Description
iM 8 User Manual -- 7 --
1.4.2 Power and Interface specifications
Power and Interface specifications
Power Supply
100~240VAC(±10%), 50/60Hz(±3Hz), 2A, 40VA Max.
Internal Battery Input
6.4V~8.4V, 2200mAh, Rechargeable Lithium battery
External DC Input
9V~15V, Vehicle or DC power supply
Display
LED+3.2" TFT LCD (320×240)
Input interface
ECG, RESP, NIBP, SPO2,TEMP and EtCO2
Output interface
Printer port, Network port
Data Storage
Support SD card
For detailed parameters, please refer to appendixA.
1.4.3 Environment Requirements
Parameters
Specification
Runtime Environment Requirements
Temperature
5 ~ 40°C (41°F ~ 104°F)
Relative humidity
-condensing)
Air pressure
70kPa ~ 106kPa
Other
Drafty and without corrosive gas
Transportation and Storage Environment Requirement
Temperature
-40°C ~ 55°C (-40°F ~131°F)
Relative humidity
-condensing)
Air pressure
16.5kPa ~ 106kPa
Other
Drafty and without corrosive gas
1.4.4 Size and Weight
Instrument
Size
180mm ×250mm ×180mm
Weight
2.0 kg (with battery)
Package
Size
310mm ×320mm ×235mm
Weight
3.7 kg
1.4.5 Related instrument
Rollstand: The manufacturer can provide different specifications of rollstand and the patient monitors on the
rollstand can be convenient to move.
Support of ward bed: the support can make the patient monitors can be very good fixed.

General Description
-- 8 -- iM 8 User Manual
1.5 Conformance Information
1.5.1 EMC
The equipment complies with all applicable and required standards for electromagnetic interference.
The safety and efficacy had been certified by the sold monitors. Though standards that the monitors accords with
may not accord to the monitors had sold, the safety and efficacy do not be weakened.
For more detailed information about EMC, please refer to appendix B of this user manual.
1.5.2 Harmonized Standards
This Patient Monitor complies with the following harmonized standards:
EN 60601-1, EN 60601-1-2,
EN 60601-1-6, EN 60601-1-8,
EN 60601-2-27, EN 60601-2-30, EN 60601-2-49,
EN 1060-1, EN 1060-3, EN 1060-4,
EN ISO 9919, EN 12470-4, EN 12470-5,
EN ISO 21647, EN ISO 10993-1,
EN 62304, EN 62366,
EN 980, EN 1041,
AAMI SP10, AAMI EC13,
ASTM E1112,ASTM E1104-03
For more information, please contact the manufacturer or the manufacturer

Installation
iM 8 User Manual -- 9 --
Chapter 2 Installation
Warning:
The instrument must be used under the direction of a professional medical person.
mable or caustic gas.
Avoid liquid or electric conducting material entering the instrument.
The instrument useAC110V~240V, user must confirm the power before plugging into the power socket.
The instrument must be connected to ground correctly and reliably.
All the non disposable accessories must be sterilized before it is used for the next patient to prevent cross
infection.
2.1 Instrument Installation
2.1.1 Unpacking and Checking
Take out the instrument from the package carefully. Check the appearance first. If there are damages due to
transportation such as the monitor is damaged, the LCD panel is broken, or there is abnormal sound when shaking
the instrument, please do not plug into the power socket or try to open the instrument to examine or repair. Instead,
contact with the local dealer or the customer service department of the manufacturer as soon as possible.
2.1.2 Installation of Support of ward bed
If needed, please contact the manufacturer or the manufacturer , the manufacturer will be
responsible for installation.
The specific methods of installation please contact the manufacturer or the manufacturer
2.1.3 Installation of Rollstand
If need, please contact the manufacturer or the manufacturer gent, the manufacturer will be
responsible for installation.

Installation
-- 10 -- iM 8 User Manual
2.1.4 ConnectingAccessories
If the instrument looks well, put it on the flat desk or fix it on the bracket. Please insert the ECG cable, NIBP cuff,
SPO2probe, TEMP probe and EtCO2probe into the corresponding socket on the right panel. If one of the
parameters is not wanted, the probe or cable could be taken off from the side panel.
If the monitor is configured with a Recorder, please install the thermal printing paper before use the Recorder.
If the monitor is configured with an external internet communication module, please connect the module to the
communication port.
2.1.5 Connecting Power Source
Connect one side of the power wire to the power socket on the backside of the instrument and the other side to the
output of AC power supply. Please do remember to confirm that the AC power is suitable for the instrument to
avoid damage of the instrument.
If the instrument is configured with an internal battery supply, the instrument can operate on the internal power
power supply. When the AC power supply is connected, the instrument uses the
AC power supply and the internal battery is charged by the AC power.
Now the instrument is ready to be used.
2.2 Description of the Panel
2.2.1 Front Panel
Figure 2.1 shows the front panel which including two parts: LED display area and LCD display area. The LEDs
are used to show the values of main physiological parameters, including NIBP (SYS, DIA, MAP), pulse rate /
heart rate, temperature, SPO2and the type of patient (adult, pediatric or neonate). The LCD is used to display
waveform, NIBP data table, EtCO2data, operating menu, system state etc. Below the panel there are several
function buttons: on-off button, printing button, NIBP measuring button, control knob, waveform freeze button,
audio control button and multi-function key.
On the left side of the LCD there are four indicators: AC indicator, DC indicator (vehicle power), charging
indicator and power on indicator.
On the right side of the LCD there are four other indicators: the alarm indicator, printing indicator and NIBP
measuring indicator and freezing indicator.
Table of contents
Other Biocare Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual