BIOFLEX Therapist User manual

01
Operation Manual
Therapist, Therapist Pro, Home Unit
THERAPIST
Therapist Operation
Manual
Safety, Installation and Operation
Instructions
Meditech International Inc.

II
Meditech International Inc.
411 Horner Ave. Unit 1
Toronto, Ontario
Canada M8W4W3
Meditech’s EU Authorized Representative:
EMERGO EUROPE
Molenstraat 15
2513 BH, The Hague
The Netherlands
Phone: +31.70.345.8570
Fax: +31.70.346.7299
Disclaimer: Meditech International Inc. adheres to recommended
safety standards and proven treatment outcome methodologies. The
company has a comprehensive training program that health care
professionals must follow, in order to properly apply Low Intensity Laser
Therapy (LILT). Meditech International does not support the incorrect
application of LILT.
Copyright © 2014 Meditech International Inc. All rights reserved.
No part of this publication may be reproduced, translated into another
language, stored in a retrieval system, or transmitted, in any form
or by any means, electronic, mechanical, photocopying, recording, or
otherwise without the prior written consent of Meditech International
Inc.
Every precaution has been taken in the preparation of this publication.
Meditech assumes no responsibility for errors or omissions. Neither is
any liability assumed for damages resulting from the use of the
information contained herein.
All brand and product names mentioned are used for identification
purposes only and are trademarks or registered trademarks of their
respective holders.
Published:
1st Edition March 2013
2nd Edition April 2014
3rd Edition October 2014
4th Edition March 2015
5th Edition June 2015
Therapist Operation Manual DOC 100.652
II
Email: info@biolexlaser.com
Web: www.biolexlaser.com
(416) 251-1055
(888) 557
-4004
(416) 251-2446
Tel:
Fax:

III
Mission Statement
III
Meditech International Inc.
initiated research in
the
development of Low
Intensity
Laser Therapy
Systems in 1988.
The goal of the company has
been to engineer
devices
that are superior
to those in
existence and
scientifica
lly develop
accurate, effective
protocols
to treat a
variety
of medical
conditions in
the
musculo-
skeletal field.
These include sports injuries
repetitive motion syndrome
and the arthritides.
To date, we have surpassed our
initial goals and expectations
but each day advances our
horizons as the educational
process continues.
Our primary objectives are
to significantly improve/
cure the multiple pathologies
that we encounter in the
shortest possible period of
time. Guidelines to that end
consist of the elimination of
pharmaceuticals and pain and
the restoration of mobility
and quality of life.

IV
Important Safety Instructions
The following safety guidelines protect your system from potential
damage and ensure personal safety:
• The system can be used in any location in the world which
provides the following AC power ratings:
1. 115 volts (V)/60 hertz (Hz) in most of North and South
America and some Far Eastern countries such as Korea
and Taiwan using the MPU30A-1-1 AC adapter, or
2. 100V/50Hz in eastern Japan and 100V/60Hz in
Western Japan using the MPU30A-1-1 AC adapter, or
3. 230V/50Hz in most of Europe, the Middle East, and
the Far East using the MPU30A-1-1 AC adapter.
• AC adapters are available by contacting Meditech or your
local distributor.
•To help prevent electric shock, plug the Controller Unit (CU)
into properly grounded power sources. For best grounding,
plug into a receptacle marked “Hospital Grade.”
• Using extension cords with the system might affect its
performance
• All system cables should be periodically inspected for signs
of deterioration. If damaged, contact Meditech’s authorized
service centers to repair or replace the cable(s).
• Do not pull, kink or pinch the cables. All cables should be
loosely wound in a loop and they should never be tightly
wrapped around the Treatment Array.
• Never remove the ground pin of the AC power cord.
IV

V
• Caution: Use of controls or adjustments or performance of
procedures other than those specified herein may result in
hazardous radiation exposure.
• Be sure nothing rests on your system’s cables and that the
cables are not located where they can be interfered with.
• Only properly trained personnel should use the system.
• Do not spill food or liquids on or in your system.
• Keep system away from radiators and other heat sources.
• If the system is not in use, you should protect it against
unqualified use by activating the key lock in the CU’s Main
Menu screen.
• To reduce the risk of electric shock, do not remove covers from
any part of the system. There are no serviceable parts inside.
Refer servicing to Meditech’s qualified service personnel.
• Wear safety glasses when using the LD-Series Treatment
Heads. Meditech recommends wearing safety glasses for all
Treatment Heads at all times.
• In case of emergency, press the RED “LASER STOP” button
•On the front of the CU to immediately stop the emission of
light radiation and to stop the treatment.
• The LD-Series Treatment Heads should only be used by
qualified personnel. The LD-Series Treatment Heads should
never be provided for patient use.
• The LD-Series Treatment Heads (lasers) should be protected
against unauthorized use by using the ‘System Lock’.
V

VI
•Some Treatment Arrays require cooling after
use. See the section “Treatment Array Cooling” in
the “LS-Series Treatment Arrays and SLD-Series
Treatment Arrays” section under “System
Features, Indicators and Controls” for information
about Treatment Array cooling.
•Care should be taken when operating the system
as external surfaces of the treatment heads,
controller and power adapter may become hot.
•Before treating, always disconnect the system
from any connection to a computer.
•The system should be installed and operated
according to CAN/ CSA-Z386: 2001 “Laser Safety in
Health Care Facilities.”
•Equipment should not be used adjacent to or
stacked with other equipment and that if
adjacent or stacked use is necessary, the
equipment should be observed to verify normal
operation in the configuration in which it will be
used.
•Equipment must not be disposed of as unsorted
municipal waste, but must be disposed of and
collected separately.
•Although Meditech has taken all reasonable
precautions to ensure system safety, it is also
the responsibility of the therapist to ensure their
personal safety.
•For Contraindications, please refer to the
Clinical User’s Manual.
VI

VII
Rules on Laser Safety (LD-Series)
Lasers of the LD-Series Treatment Heads produce a very intense
beam of light that is potentially hazardous if the direct beam
or specular reflection is viewed by the unprotected eye. The
following precautions should be taken to avoid direct beam
viewing and diffraction. Read the provided “Laser Safety Guide”
from the Laser Institute of America prior to using the system.
•Make certain that your system is located and operated
in a controlled area when using the LD-Series Treatment
Heads. Remove all shiny objects from the area in which
you will be working. This includes rings, watches, metal
bands, tools, and glass. Reflections from the beam can
be nearly as intense as the beam itself.
•The entrances to the area should be posted with the
provided laser danger sign.
•Never look directly into the laser beam or stare
at its reflections. OCULAR DAMAGE CAN OCCUR.
•Never use magnifiers (such as binoculars or
telescopes) to look at the beam.
•Never point the laser at the eyes directly.
•If you are applying the laser to a region of the face,
please ensure that the patient is wearing the safety
eye shields which protect against possible exposure of
the eye to the laser beam.
•Never leave a laser unattended while it is turned on.
•Wear safety glasses! Every person within the enclosed
area must wear safety glasses or eye shields when using
the LD-Series Treatment Head.
•Specific labeling is required on all laser products. For
safety purposes, do not remove any of the labels.
VII
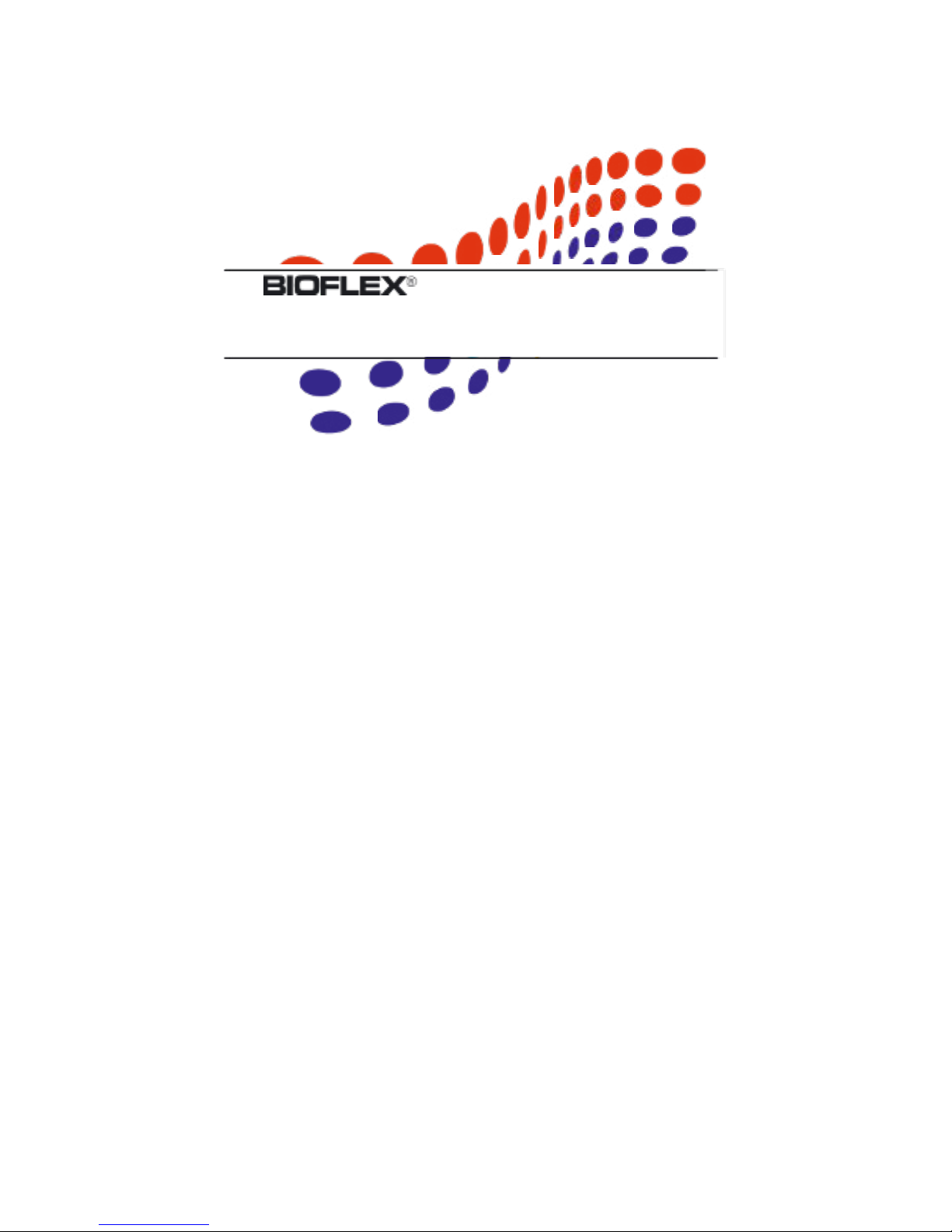
VIII
Important Notice for the LD-Series
Treatment Heads
When applying the Treatment Head, ensure that the entire
nosecone tip (also called “body switch”) has complete contact
with the skin and is not applied at an angle. Failure to do so
will allow potentially harmful laser light to irradiate surrounding
areas.
Correct Application Incorrect Application
Figure 1 LD-Series Treatment Head Application
Safety Glasses Use
FAILURE TO FOLLOW THESE INSTRUCTION MAY
RESULT IN SERIOUS INJURY AND/OR BLINDNESS
READ ALL INSTRUCTIONS AND WARNINGS
BEFORE USING THE SYSTEM
• Never use the safety glasses (or eyewear) with other
manufacturer’s equipment.
• Never use other manufacturer’s safety glasses with the BioFlex
system.
VIII

IX
•Always avoid direct eye exposure to direct or reflected
laser light.
•Ensure that the safety glasses it securely at all times.
•Always check safety glasses before use to assure that
they have not become damaged. Exposure to or
contact with chemical vapors or liquids may also cause
surface cracks or other damage. Do not use if you observe
any type of damage to your eyewear and replace your
eyewear immediately.
•Always keep your safety glasses in the Therapist
carrying case when not in use.
•Never use the safety glasses for any purpose other
than for eye protection while using the system.
Cleaning Instructions
Between patient therapy sessions as indicated
•Wash in mild soap and water.
•Rinse in clean water.
•Air dry or pat dry with a clean, soft tissue.
IX
DO NOT USE AMMONIA, ALKALINE CLEANERS,
ABRASIVE CLEANING COMPOUNDS OR
SOLVENTS
WHEN CLEANING SAFETY GLASSES.
CERTAIN SOLVENTS MAY LOWER THE IMPACT
RESISTANCE OF THE LENSES.

X
Safety Kit
The BioFlex Safety Kit consists of the following:
• Laser Safety Guide
• Laser Danger Sign
• Two pairs of safety glasses (one for the practitioner and one
for the patient)
Please read the Laser Safety Guide. Post the danger sign outside
the entrance to the room where the system is located. If
there is
more than one entrance into the room where the system is
located
, please contact Meditech to obtain additional signs
to
post in these areas.
X

XI
Table of Contents
Important Safety Instructions
Rules on Laser Safety
Safety Glasses Use
Safety Kit
Classification
Safety Standards
Electromagnetic Compatibility (EMC)
Explanation of Warning Symbols and Labels
Controller Unit (CU)
Classification Marks
LD-Series Treatment Head Labels
LS-Series Treatment Head Labels
SLD-Series Treatment Head Labels
DUO+Treatment Head Labels
Description of the Beam Delivery Systems
LS-Series Treatment Heads
LD-Series Treatment Heads
iv
vii
viii
x
13
14
15
16
16
16
17
21
25
25
27
27
27
XI

XII
XII
System Features, Indicators and Controls
Controller Unit
LS-Series Treatment Arrays
Applying and Removing Treatment Heads
LS-Series
LD-Series
Operation
Environmental Conditions
Calibration
Cleaning
If the System Becomes Damaged
Guidelines for Proper Packaging
Error Messages
Specifications
Controller Unit
Treatment Heads
Glossary
System Parts List
29
29
34
37
38
39
41
54
55
56
57
58
59
63
63
64
66
67

13
Classification
The system is classified as a medical device. Furthermore, it is in
accordance to the Sub-clause 6 of IEC 60601-1 and Clause 6 of
IEC 60601-1:2005:
•CLASS I EQUIPMENT, according to the type of
protection against electric shock;
•TYPE B APPLIED PART, according to the degree of
protection against electric shock;
•NOT CLASSIFIED, according to the degree of
protection against harmful ingress of water;
•CONTINUOUS OPERATION, according to the mode
of operation;
•NONE OF THE PARTS OF THE SYSTEM ARE STERILIZED.
13

14
Safety Standards
This product is classified as medical equipment and has been
tested to comply for the following standards:
Canadian standards
• CAN/CSA C22.2 No. 60601.1, Third ed. -Medical Electrical
Equipment -Part 1: General Requirements for Safety
• CAN/CSA C22.2 No. 601-1.M90 -Medical Electrical Equipment
–Part 1: General Requirements for Safety
• Canadian and United States of America standards
• UL 60601-1, First ed. -Medical Electrical Equipment -Part 1:
General Requirements for Safety
• 21 CFR 1040 -Performance Standards for Light-Emitting
Products
International standards
• IEC/EN 60601-1, Second ed. -Medical Electrical Equipment -
Part 1: General Requirements for Safety
• ANSI/AAMI ES60601-1:2005 -Medical electrical equipment
-Part 1: General requirements for basic safety and essential
performance
• IEC 60601-1-2 - Medical Electrical Equipment -Part 1:
General Requirements for Safety -2. Collateral Standard:
Electromagnetic Compatibility -Requirements and Tests
• IEC 60601-2-22:2007 -Medical Electrical Equipment -Part
2: Particular Requirements for the Safety of Diagnostic and
Therapeutic Laser Equipment
• IEC 60825-1 - Safety of Laser Products -Part 1: Equipment
Classification, Requirements and User’s Guide
14

15
Electromagnetic Compatibility (EMC)
This equipment complies with the European rules for EMC
according to the safety standard IEC 60601-1-2. This device
complies with EMC rules under test conditions that include use
of system cables and connectors between system components.
This Equipment requires special precaution regarding the EMC
and must be installed and put into service according to the
information provided in this manual.
The use of accessories and cables other than those specified and
sold by the manufacturer may result in increased emissions or
decreased immunity of the equipment and may cause the system
to be non-compliant with the requirements of IEC 60601-1-2
Replace cables and connectors between components only with
Meditech approved cables and connectors.
This system may cause radio interference or may disrupt the
operation of nearby equipment. It may be necessary to take
mitigation measures, such as reorienting or relocating the
equipment or shielding the location.
15
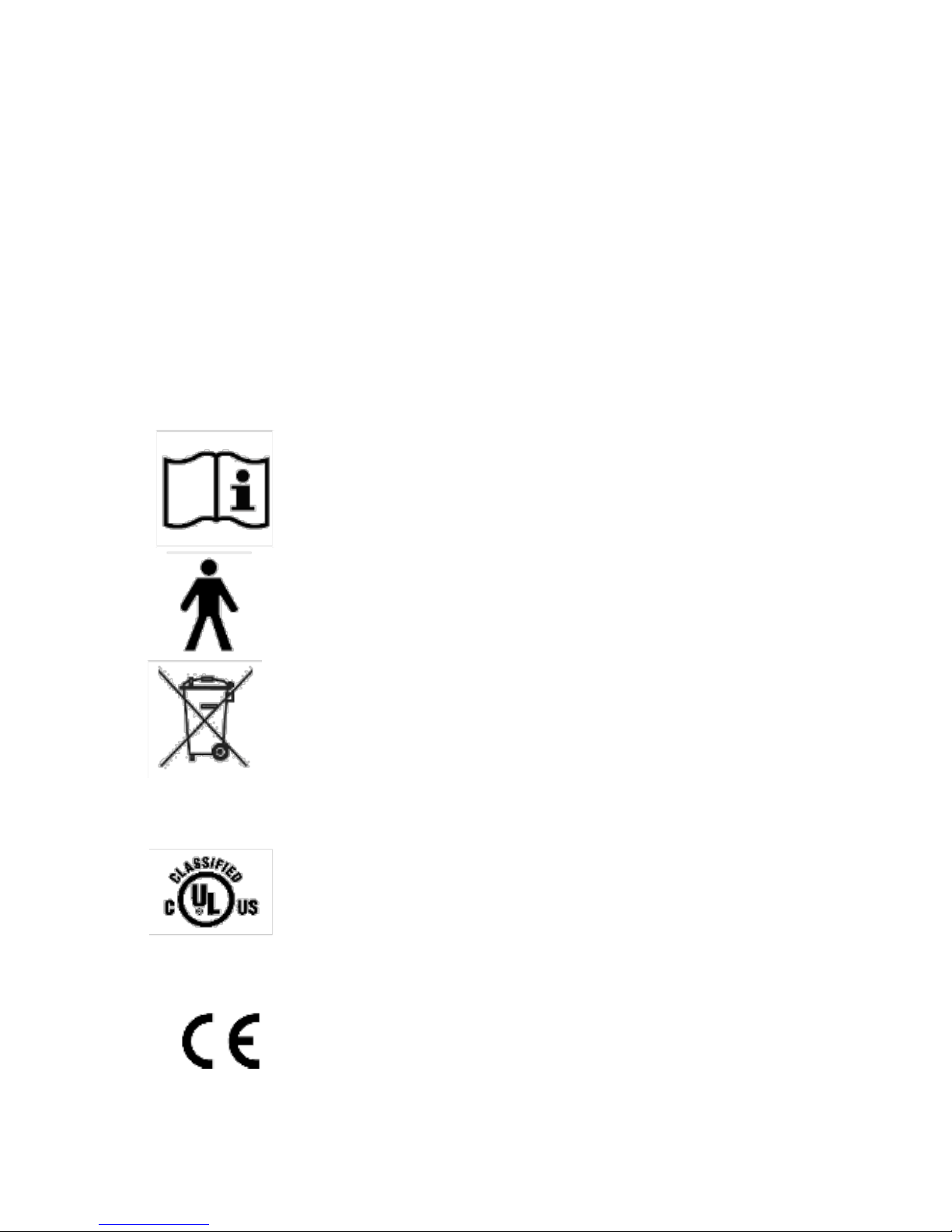
16
Explanation of Warning Symbols and Labels
This section explains the warning symbols and labels on the
CU and the Treatment Heads. Each Treatment Head has labels
which are located on both sides (underside and topside) of the
Treatment Head.
“Grounding reliability can only be achieved when the
equipment is connected to an equivalent receptacle marked
‘Hospital Grade’.”
Controller Unit (CU)
The CONSULT OPERATION MANUAL symbol.
Parts of the system are marked with this warning
symbol when it is necessary for the user to refer
to the instructions in the Operation Manual.
The TYPE B APPLIED PART symbol. This product
is classified as a Type B Applied Part, according to
the degree of protection against electric shock.
This symbol indicates that the waste of electrical
and electronic equipment must not be disposed
as unsorted municipal waste and must be
collected separately.
Classification Marks
The UL mark is a registered trademark of the
Underwriters Laboratories Inc. with respect
to electric shock, ire, mechanical and other
specified hazards only in accordance with CAN/
CSA C22.2 No. 601.1, CAN/CSA C22.2 No.
601.2.22 and UL 60601-1.
The CE mark is a registered trademark of the
European Community
16

17
LD-Series Treatment Head Labels
Figure 2 LD-I 75 Treatment Head Explanatory Label
Figure 3 LD-I 200 Treatment Head Explanatory Label
17

18
Figure 4 LD-R 100 Treatment Head Explanatory Label
“VISIBLE LASER RADIATION” or “INVISIBLE LASER RADIATION”
Laser (LIGHT AMPLIFICATION BY STIMULATED EMISSION OF
RADIATION) is a device which can be made to produce or amplify
electromagnetic radiation in the wavelength range from 180
nm to 1 mm primarily by the process of controlled stimulated
emission. For the LD-series Treatment Heads, the laser light is
radiated from the top of the Treatment Head nosecone tip (see
section “Description of the Beam Delivery System”).
“AVOID EXPOSURE TO BEAM”
Never look directly into the laser beam or stare at its bright
reflections which are radiated from the aperture of the
Treatment Head.
“CLASS 3B LASER PRODUCT”
This “CLASS 3B LASER PRODUCT” emits laser light at a wavelength
where direct beam viewing is hazardous. Avoid viewing diffuse
reflections.
18
19
For this “CLASS 3B LASER PRODUCT” eye protection is required
and the system has to be operated in an enclosed area where the
entrance should be posted with the provided laser warning sign.
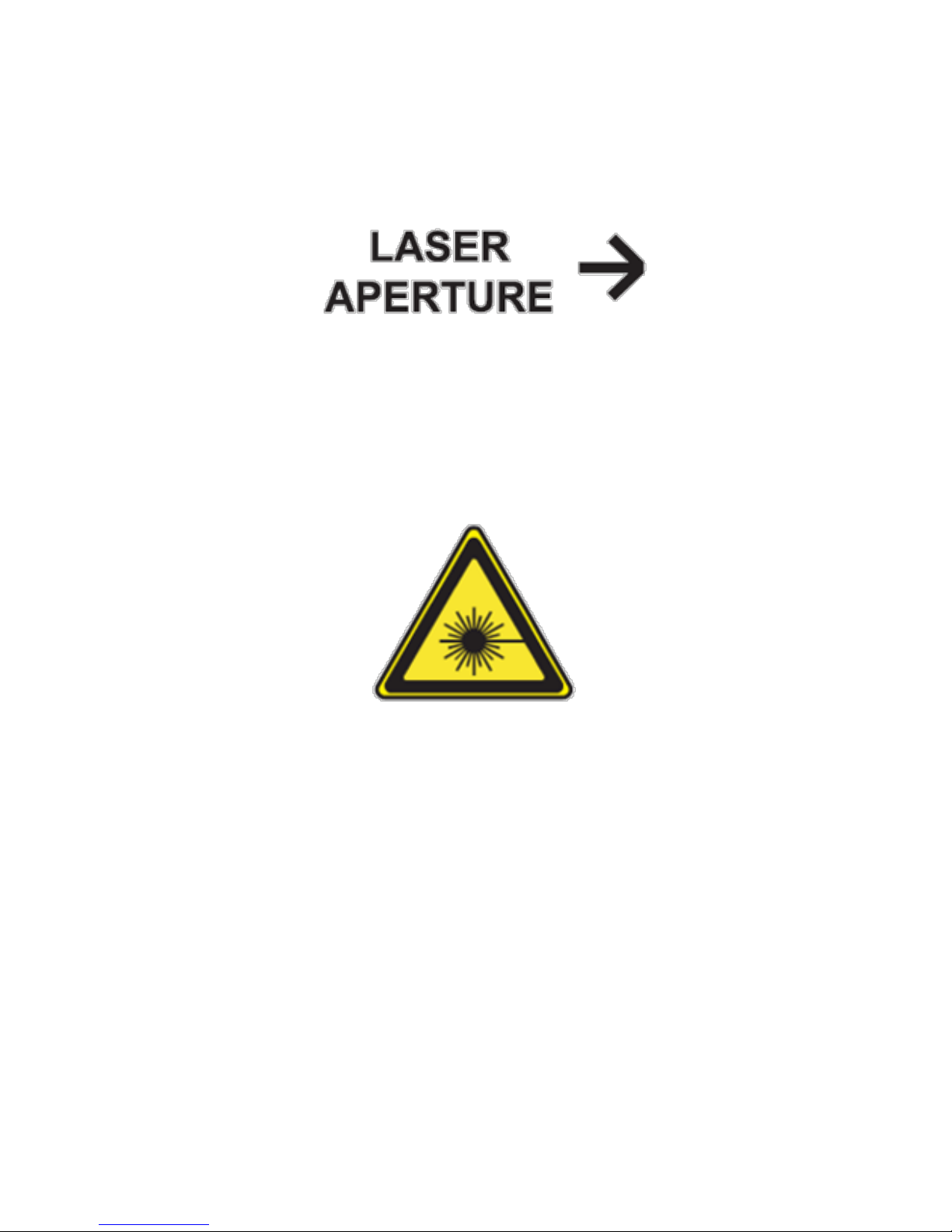
Figure 5 LD-Series Treatment Head Laser Aperture Label
“LASER APERTURE”
This label shows the location of the aperture (LD-series Treatment
Head tip) through which the laser radiation is emitted. Please see
“Description of the Beam Delivery System”.
Figure 6 LD-Series Treatment Head Hazard Symbol
“YELLOW TRIANGLE”
This is a hazard label warning that the system emits hazardous
light. See “CLASS 3B LASER PRODUCT.”
This declaration is required by the U.S. Food and Drug
Administration (FDA) in accordance with the standard 21 CFR
1040 -Performance Standards for Light-Emitting Products.
“This product is in conformity with performance standards for
laser products under 21 CFR 1040, except with respect to those
characteristics authorized by Variance Number 98V0816/7
effective October 7, 1998.”
19
This manual suits for next models
2
Table of contents
Other BIOFLEX Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual