BIOFLEX Arthritis User manual
www.bioexlaser.com
BIOFLEX® Arthritis

2MN421.018 Rev 3
Copyright © 2022 Meditech International Inc. All rights reserved.
Manufactured by: Meditech International Inc.
No part of this publication may be reproduced, translated into another
language, stored in a retrieval system, or transmitted, in any form or by
any means, electronic, mechanical, photocopying, recording, or otherwise
without the prior written consent of Meditech International Inc.
Every precaution has been taken in the preparation of this publication.
Meditech assumes no responsibility for errors or omissions. Neither is any
liability assumed for damages resulting from the use of the information
contained herein.
All brand and product names mentioned are used for identication
purposes only and are trademarks or registered trademarks of their
respective holders.
BIOFLEX®Arthritis System User Manual
Date of issue: – Jan 26, 2022
Meditech International Inc.
411 Horner Ave., Unit 1
Toronto, ON,
Canada M8W 4W3
Toll Free: 1-844-770-0177 (North
America)
Phone: 1-416-251-1055;
1-888-557-40044Ext: 117 & 137
Email: service@bioexlaser.com
Website: www.bioexlaser.com
The BIOFLEX®Arthritis System

MN421.018 Rev 3 3
Table Of Contents
Introduction 4
System Basics 5
Planning Your Treatments 6
Essential Performance 8
Conditions Treated and Placement of Array 9
What’s In The Box 22
Connecting The System 23
Application Of Treatment 26
Important Information 27
System Troubleshooting 29
System Specications 31
Safety Standards 32
Maintenance and Repair 34
Electromagnetic Compatibility (EMC) 35
BIOFLEX®Arthritis System Parts List 40
Notes 41

4MN421.018 Rev 3
Introduction
The BIOFLEX® Arthritis system, for the rst time in the history of the
application of light therapy, has the capacity to deliver automated, pre-
programmed treatment protocols for an extensive number of joints
aected by arthritis. The system can be easily applied at home, while
traveling or otherwise engaged.
The unit is a highly sophisticated derivative product of the BIOFLEX®Series
of Professional Laser Therapy systems that have achieved global acclaim in
the mainstream medical community, based on the eective resolution of
many challenging pathologies.
Currently BIOFLEX® systems are utilized in the eld of Laser Medicine by
health care professionals worldwide. The technology is widely applicable
in the treatment of musculoskeletal conditions, arthritis, sports and soft
tissue injuries and many other both complex and routine medical problems.
At Meditech International we view this system as a major advance in
providing quick relief of pain and other symptoms. An extensive range of
medical problems can be rapidly improved -- the result of the initiation of
a cascade of physiological activities leading to the restoration of normal
cell structure and function. The system is also instrumental in providing
preventative and maintenance therapy of body tissues.
With the acquisition of this unit, you hold the key to fast and eective relief
for many of your arthritic problems, at your ngertips.
The History of Meditech International
Inc.
The design, manufacture and therapeutic application
of BIOFLEX®systems is the primary focus of the
company - founded by Fred Kahn, MD, in 1989.
Dr. Kahn is a Fellow of The Royal College of Surgeons
of Canada, a Diplomate of the American Board of
Surgery and has been elected to The Spinal Hall of
Fame for his pioneering eorts in the resolution of
back problems.
Prior to initiating research in the eld of Laser Technology, he was engaged
in a surgical practice specializing in trauma and vascular reconstructive
surgery over the course of twenty-ve years

MN421.018 Rev 3 5
During the past quarter of a century, Dr. Kahn has assembled an innovative
team of scientists, clinicians, software and electro-photonic specialists in
order to standardize and advance the eld of “Laser Medicine”.
During the rst ten years of its existence, the company was based at
Ryerson University in Toronto, Canada where it developed and veried its
initial concepts.
Dr. Kahn has published four texts on the topic of Laser Medicine, along
with numerous articles relating to this emerging technology and as a result
of this process, has become a recognized leader in this eld.
System Basics
The BIOFLEX®Arthritis system is a high performance therapy system that is
cost-eective and easy to use. The ergonomically designed Controller Unit
is connected to a exible Treatment Array in order to automatically deliver
a patented sequence of Red and Infrared light therapy to arthritic joints.
Research-based protocols have been developed for the most common
joints aected by arthritis. Four protocol stages are available to treat each
area.
The Treatment Array’s soft, exible composition readily adjusts to the
congurations of the anatomical area to which it is applied.
Each treatment is initiated by the emission of a stream of Red Light,
automatically followed by Infrared Light. The latter is not visible to the
human eye.
Once the treatment has been completed this will be conrmed by a sound
marking that event

6MN421.018 Rev 3
Planning Your Treatments
For most arthritic conditions, your treatments can take anywhere from
30 minutes to an hour, therefore we recommend nding a comfortable
location and plan something relaxing while the treatment is ongoing.
Placement of the Array
Light therapy is most eective when the Treatment Array is placed rmly on
the skin and directly over the arthritic joint (focal point of the pain). Before
commencing treatment, secure the array by using the strap provided. In
some instances a weight (beanbag) can also be used. Once your initial
treatment has cycled through RED and INFRARED light, reposition to the
next placement and start treatment again. Treat around the entire arthritic
joint using as many placements as required (refer to pages 10-21). It is
also benecial to position the Array on muscles adjacent to the arthritic
joint if they are also painful.
Stages of Treatment
There are 4 pre-programmed Stages for each of the treatment areas
depicted on the Controller Unit. For any aected area, Stage 1 should
be used initially for 2-3 weeks until you are no longer improving at which
point proceed to Stage 2. Stages 3 & 4 would be used if your pain relief
is continuing to plateau. Most often Stages 1 and 2 provide optimal relief
of arthritic symptoms.
Frequency of Application
Generally treatment is applied once daily for the rst 4-5 days. In some
instances, particularly when pain is acute, two treatment sessions may be
applied twice daily for four or more days, allowing 8-10 hours between the
two sessions. If after 5 or more treatments improvement is not signicant,
one may advance to Stage 2. Applying treatment more than twice over a
24-hour period should be avoided.
As your symptoms diminish with continuing therapy, the frequency of
treatments may be gradually reduced. Often continuing therapy once or
twice each week will continue the healing process and avoid recurrence of
symptoms. If your arthritic joints become aggravated and the pain is once
again acute, follow the initial instructions above.

MN421.018 Rev 3 7
Additional Instructions
• All areas are treated with the application of the Array, which delivers
Red light initially, followed sequentially by the Infrared automatically.
• Following the initial placement (please see diagrams) and initiation of
the treatment protocol, the rst placement will end as indicated by 3
consecutive beeps. In order to continue with treatment, now place the
Array onto the next corresponding area according to the instructions
(see pages 10-21). Press the Start/Pause button, which will initiate
treatment over the next placement. Repeat for each of the placements
required in turn.
Should you require further guidance, please call Meditech service at 1-844-
770-0177

8MN421.018 Rev 3
Essential Performance
According to IEC 60601-1 Ed. 3.1, Essential Performance is performance
of a clinical function, other than that related to BASIC SAFETY, where loss
or in an unacceptable RISK. The Essential Performance of the BIOFLEX®
Arthritis system according to IEC 60601-1 Ed. 3.1, is to not radiate harmful
levels of light to shine in the eyes of the patients, operators or bystanders.
Side Eects
The following may be experienced: pain; bruising and swelling; redness
and inammation; herpes simplex outbreaks and bacterial infections;
temporary skin lightening or darkening; darkening/lightening of tattoos;
freckle loss or lightening of moles; permanent skin pigment changes;
exacerbation of preexisting skin conditions; and hair loss at the treatment
area.
Contraindications
The use of BIOFLEX®Arthritis is contradindicated: for patients with lupus
and other diseases which may be stimulated by light, history of keloids,
recent excessive sun exposure and /or articial tanning at the treatment
area, recent patients using photosensitive medications or herbs and
patients who are pregnant. For additional advice consult your personal
physician or consultants at Meditech.
Termination of Treatment
Treatments end automatically, unless one presses the Stop button prior to
that event.
The Treatment Array may be cleaned after each treatment using an alcohol
wipe or a soft, damp cloth. A mild disinfectant may also be used. Do not
immerse or rinse the Treatment Array in water.
The straps may be cleaned in a washing machine using a gentle cycle.
Allow straps to air dry post-washing.

MN421.018 Rev 3 9
Conditions Treated and
Placement of Array
Intended Use
The BIOFLEX®Arthritis system is used by patients as well as trained health
care professionals and is indicated for use to emit energy in the red and
infrared spectrum to provide topical heating for the purpose of elevating
tissue temperature for temporary relief of arthritic pain.
Target Population
Adults (18+)
Indications For Use
The BIOFLEX®Arthritis system can be used for temporary relief of arthritis
pain. Pages 10-21 demonstrate the most common areas aected by
arthritis.
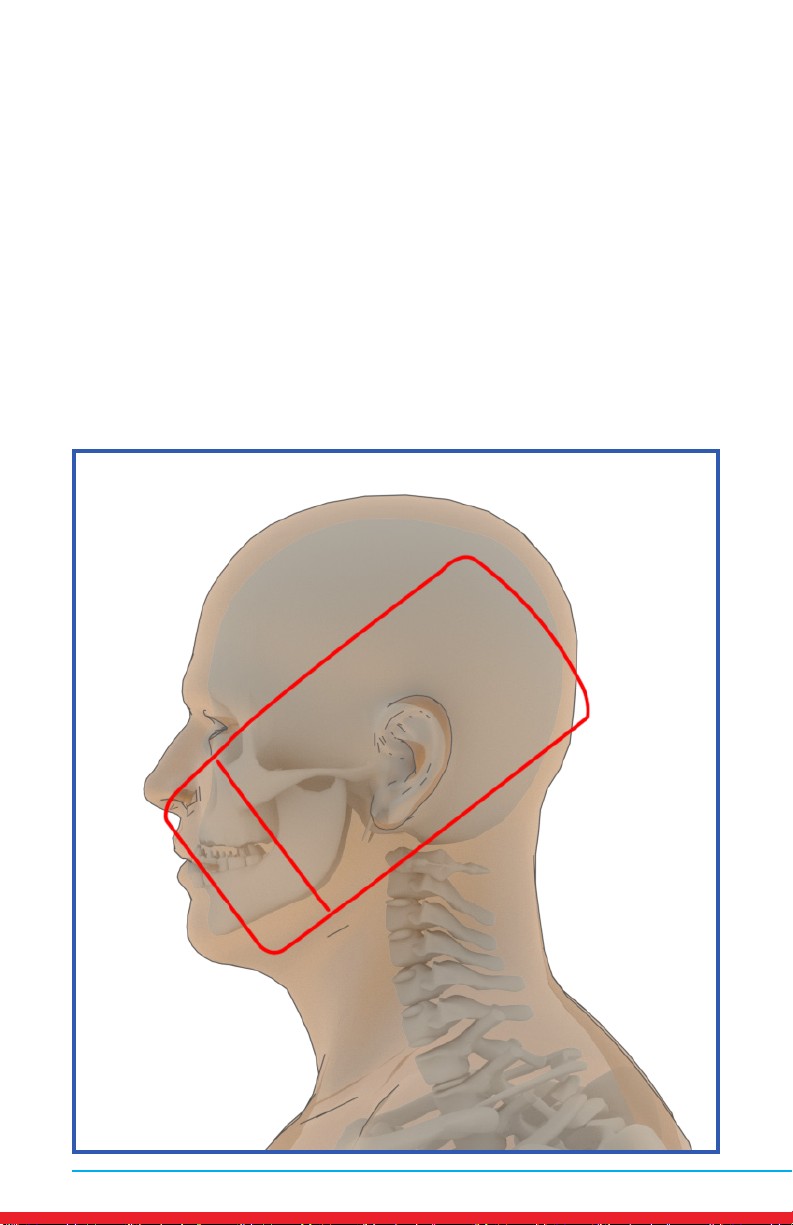
10 MN421.018 Rev 3
Temporomandibular Joint Dysfunction Pain (TMJ)
Placements
• Red outline indicates placement of Array.
• Ensure rm contact of the Array on bare skin.
• Secure the Array using provided straps or small weight
• Treat around the entire arthritic joint using as many placements as
required (refer to illustrations below).
• Position the Array on muscles adjacent to the arthritic joint if they are
also painful.
A
MN421.018 Rev 3 11
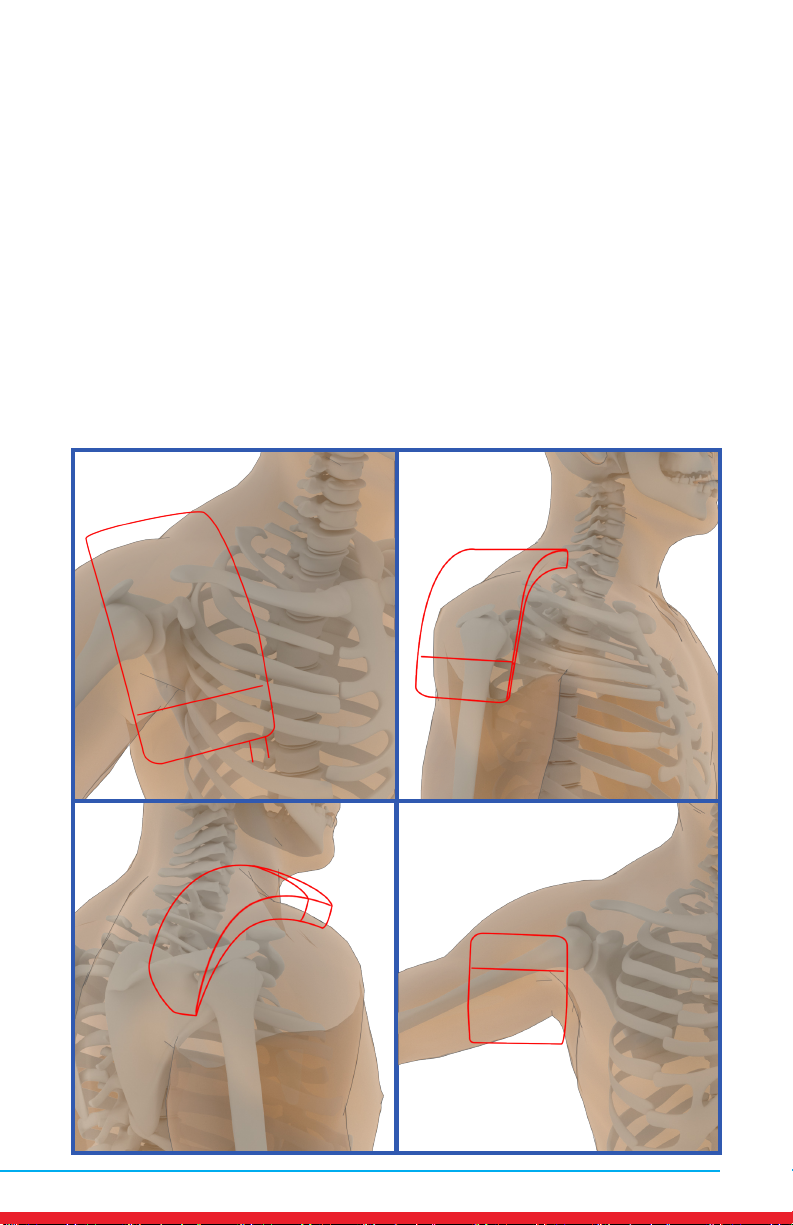
Shoulder Pain
Placements
• Red outline indicates placement of Array.
• Ensure rm contact of the Array on bare skin.
• Secure the Array using provided straps or small weight
• Once the Array cycles through RED and INFRARED light, re-position to
the next placement and start treatment again.
• Treat around the entire arthritic joint using as many placements as
required (refer to illustrations below).
• Position the Array on muscles adjacent to the arthritic joint if they are
also painful.
A B
CD
12 MN421.018 Rev 3
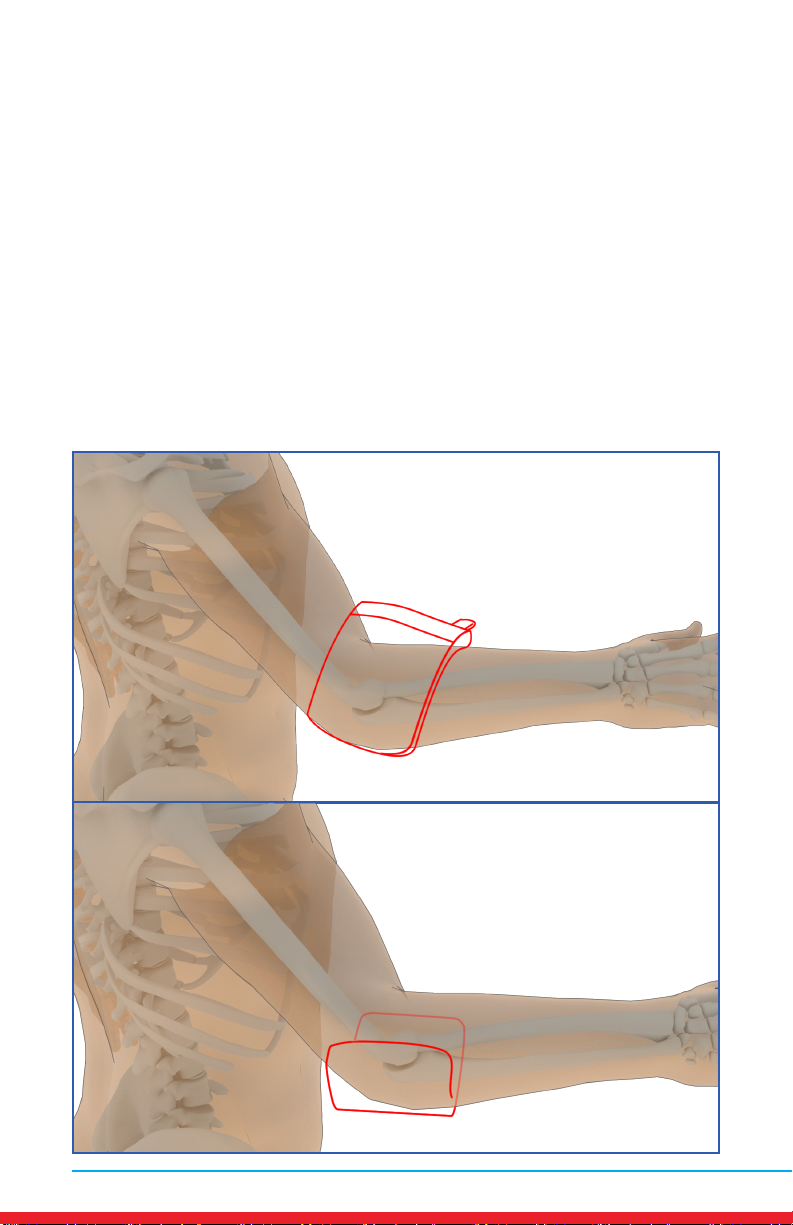
Elbow Pain
Placements
• Red outline indicates placement of Array.
• Ensure rm contact of the Array on bare skin.
• Secure the Array using provided straps or small weight
• Once the Array cycles through RED and INFRARED light, re-position to
the next placement and start treatment again.
• Treat around the entire arthritic joint using as many placements as
required (refer to illustrations below).
• Position the Array on muscles adjacent to the arthritic joint if they are
also painful.
A
B
MN421.018 Rev 3 13
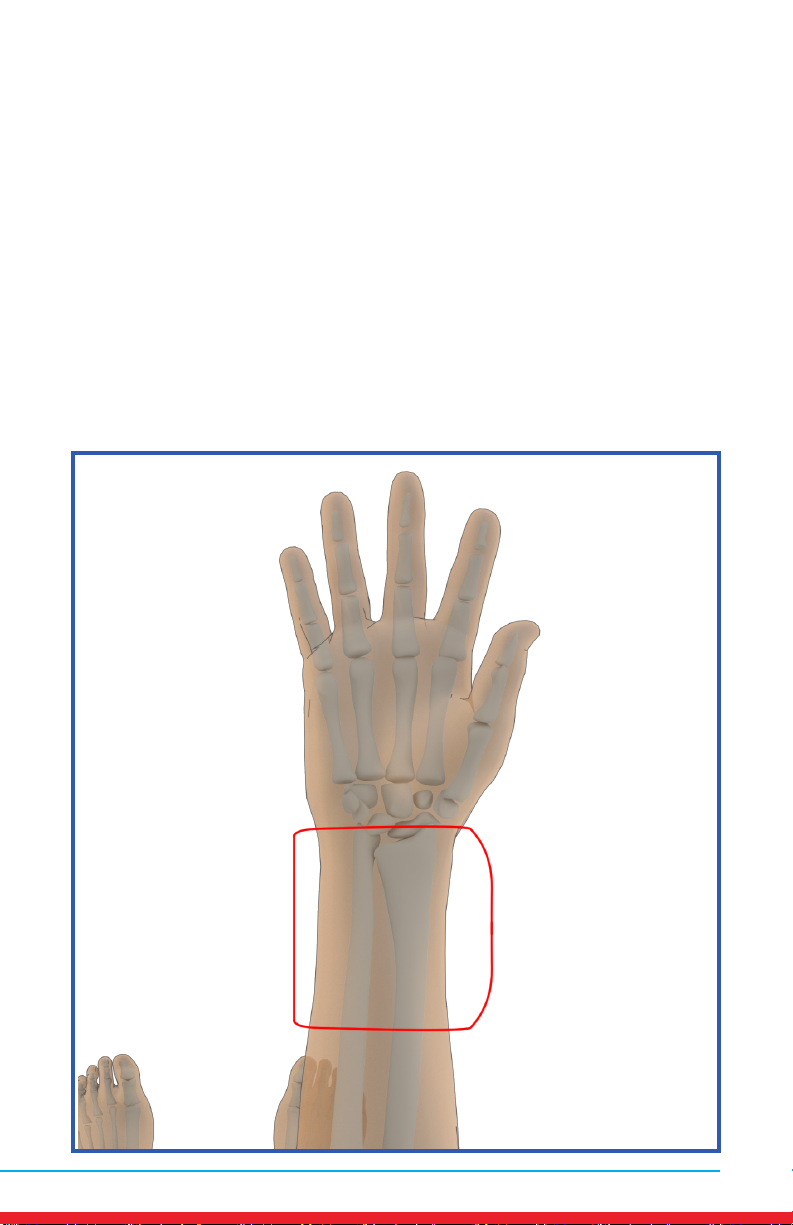
Wrist Pain
Placements
• Red outline indicates placement of Array.
• Ensure rm contact of the Array on bare skin.
• Secure the Array using provided straps or small weight
• Once the Array cycles through RED and INFRARED light, re-position to
the next placement and start treatment again.
• Treat around the entire arthritic joint using as many placements as
required (refer to illustrations below).
• Position the Array on muscles adjacent to the arthritic joint if they are
also painful.
A
14 MN421.018 Rev 3
A
C
B
D
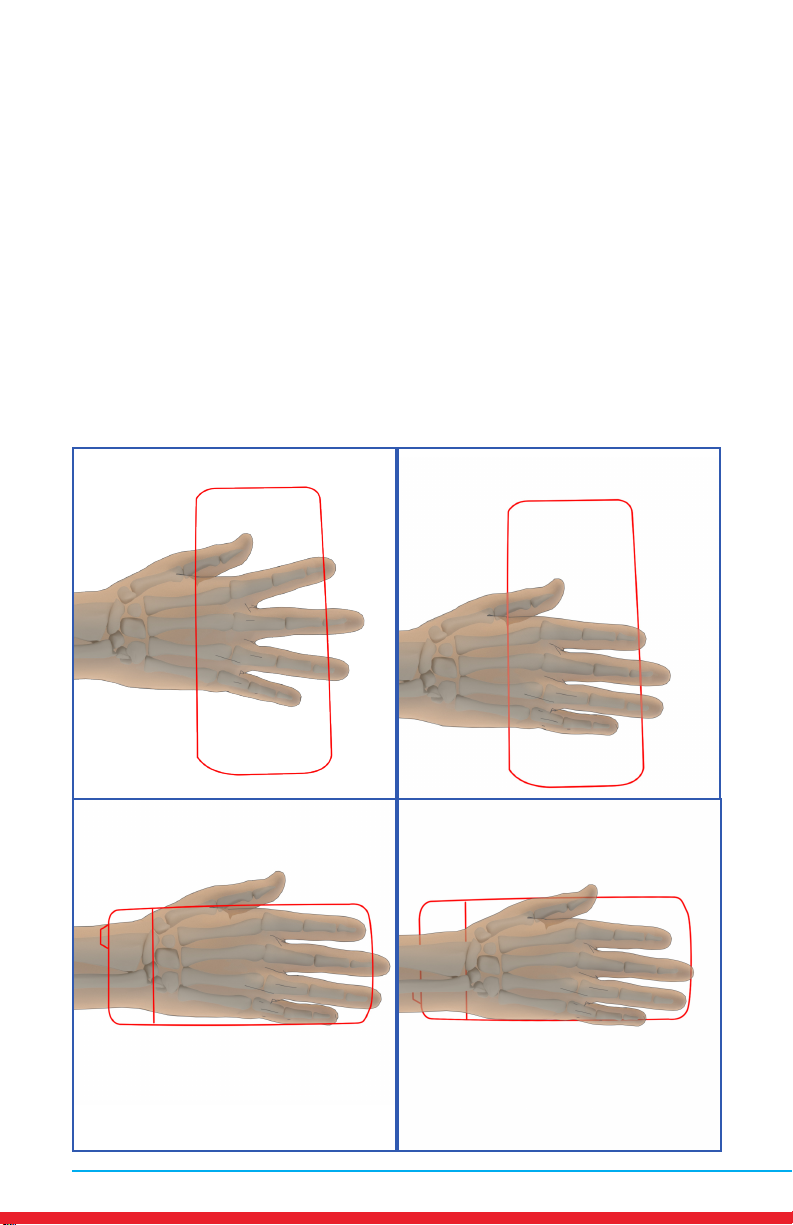
Hand Pain
Placements
• Red outline indicates placement of Array.
• Ensure rm contact of the Array on bare skin.
• Secure the Array using provided straps or small weight
• Once the Array cycles through RED and INFRARED light, re-position to
the next placement and start treatment again.
• Treat around the entire arthritic joint using as many placements as
required (refer to illustrations below).
• Position the Array on muscles adjacent to the arthritic joint if they are
also painful.
MN421.018 Rev 3 15
AB
CD
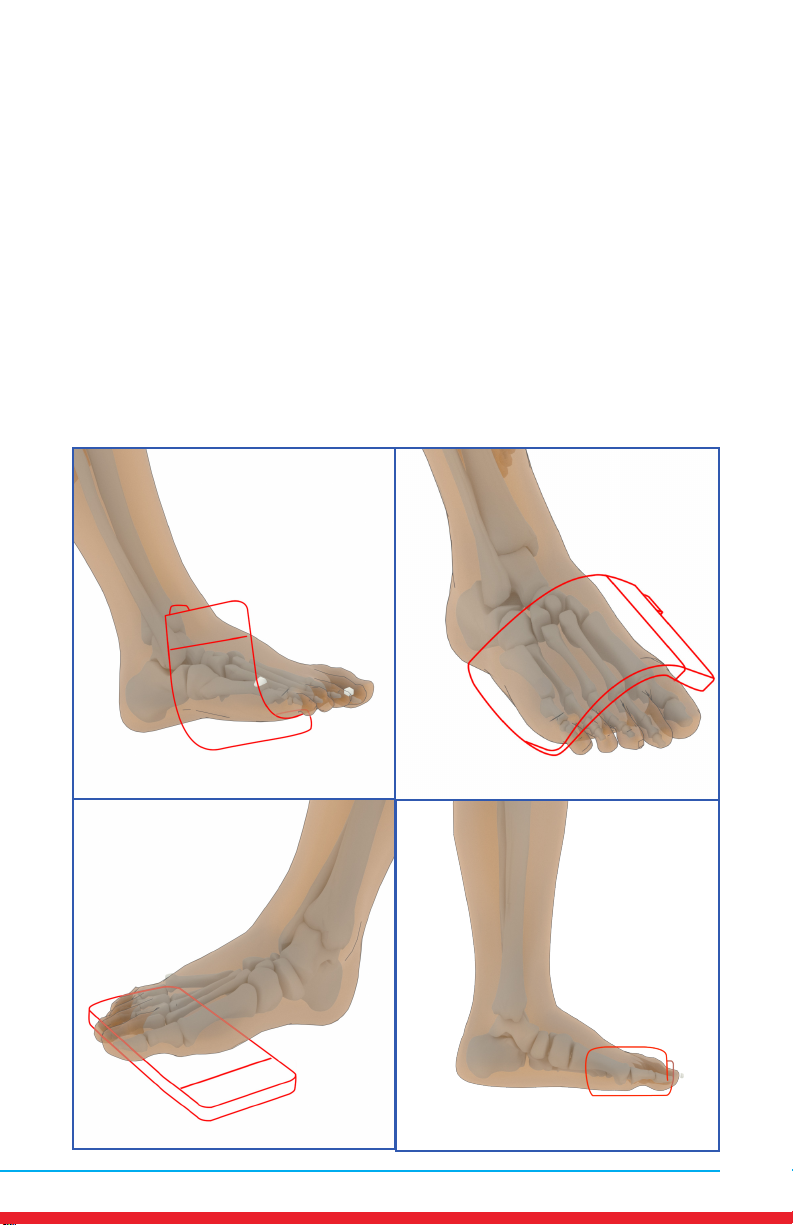
Foot Pain
Placements
• Red outline indicates placement of Array.
• Ensure rm contact of the Array on bare skin.
• Secure the Array using provided straps or small weight
• Once the Array cycles through RED and INFRARED light, re-position to
the next placement and start treatment again.
• Treat around the entire arthritic joint using as many placements as
required (refer to illustrations below).
• Position the Array on muscles adjacent to the arthritic joint if they are
also painful.
16 MN421.018 Rev 3
A
B
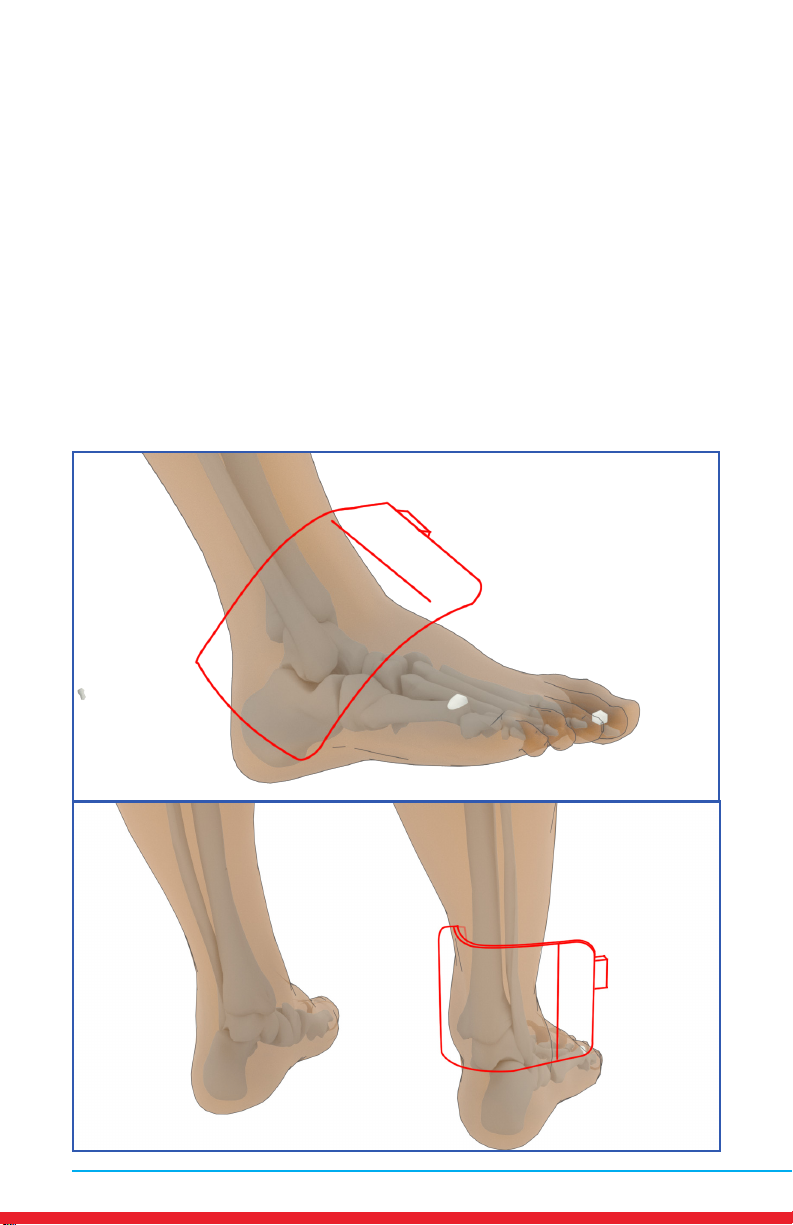
Ankle Pain
Placements
• Red outline indicates placement of Array.
• Ensure rm contact of the Array on bare skin.
• Secure the Array using provided straps or small weight
• Once the Array cycles through RED and INFRARED light, re-position to
the next placement and start treatment again.
• Treat around the entire arthritic joint using as many placements as
required (refer to illustrations below).
• Position the Array on muscles adjacent to the arthritic joint if they are
also painful.
MN421.018 Rev 3 17
AB
C D
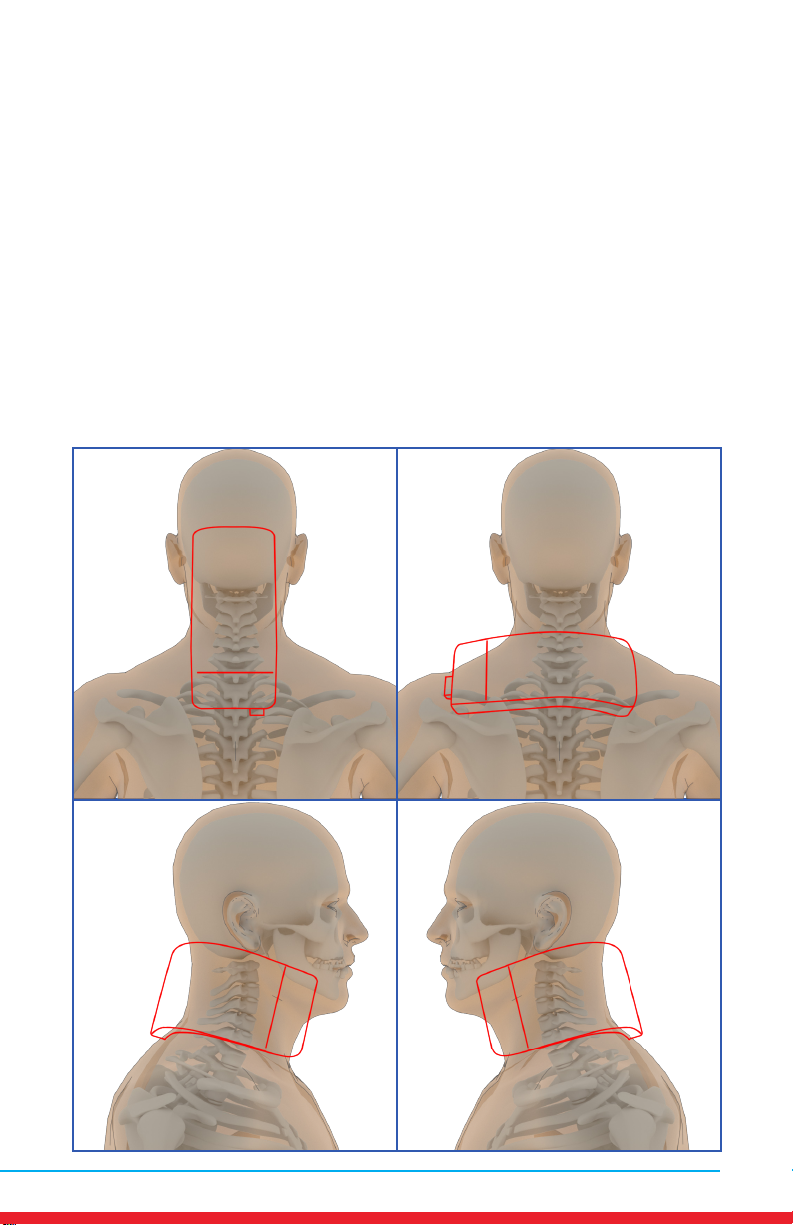
Cervical Spine Pain (Neck)
Placements
• Red outline indicates placement of Array.
• Ensure rm contact of the Array on bare skin.
• Secure the Array using provided straps or small weight
• Once the Array cycles through RED and INFRARED light, re-position to
the next placement and start treatment again.
• Treat around the entire arthritic joint using as many placements as
required (refer to illustrations below).
• Position the Array on muscles adjacent to the arthritic joint if they are
also painful.
18 MN421.018 Rev 3
A
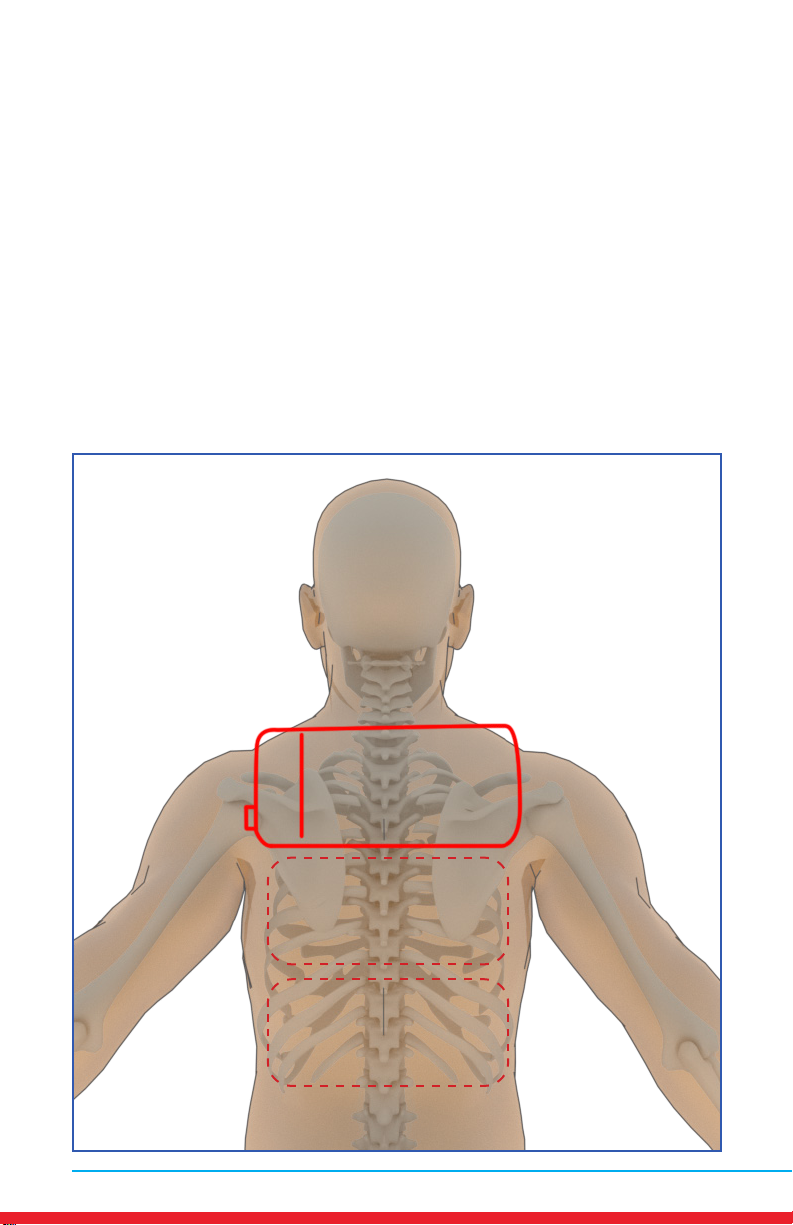
Thoracic Spine Pain (Upper Back)
Placements
• Red outline indicates placement of Array.
• Ensure rm contact of the Array on bare skin.
• Secure the Array using provided straps or small weight
• Once the Array cycles through RED and INFRARED light, re-position to
the next placement and start treatment again.
• Treat around the entire arthritic joint using as many placements as
required (refer to illustrations below).
• Position the Array on muscles adjacent to the arthritic joint if they are
also painful.
MN421.018 Rev 3 19
A
BC
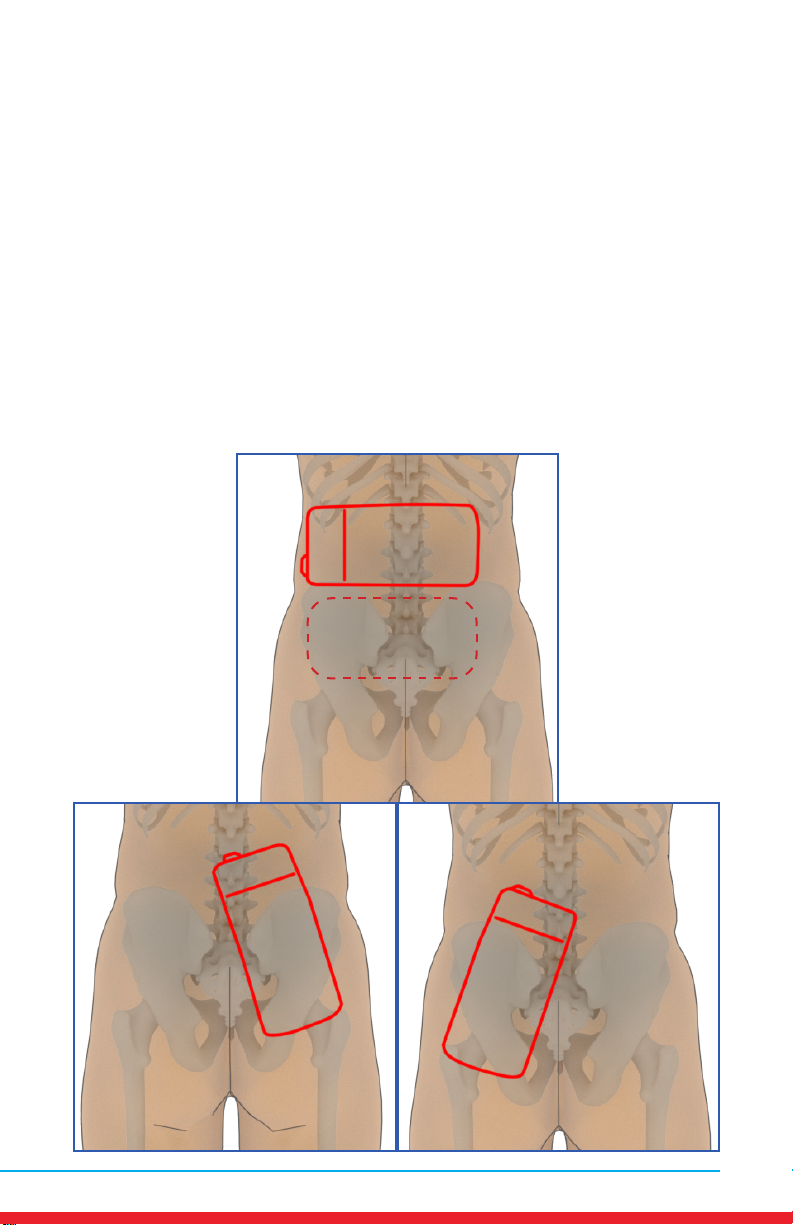
Lumbar Spine Pain (Lower Back)
Placements
• Red outline indicates placement of Array.
• Ensure rm contact of the Array on bare skin.
• Secure the Array using provided straps or small weight
• Once the Array cycles through RED and INFRARED light, re-position to
the next placement and start treatment again.
• Treat around the entire arthritic joint using as many placements as
required (refer to illustrations below).
• Position the Array on muscles adjacent to the arthritic joint if they are
also painful.
20 MN421.018 Rev 3
CB
A
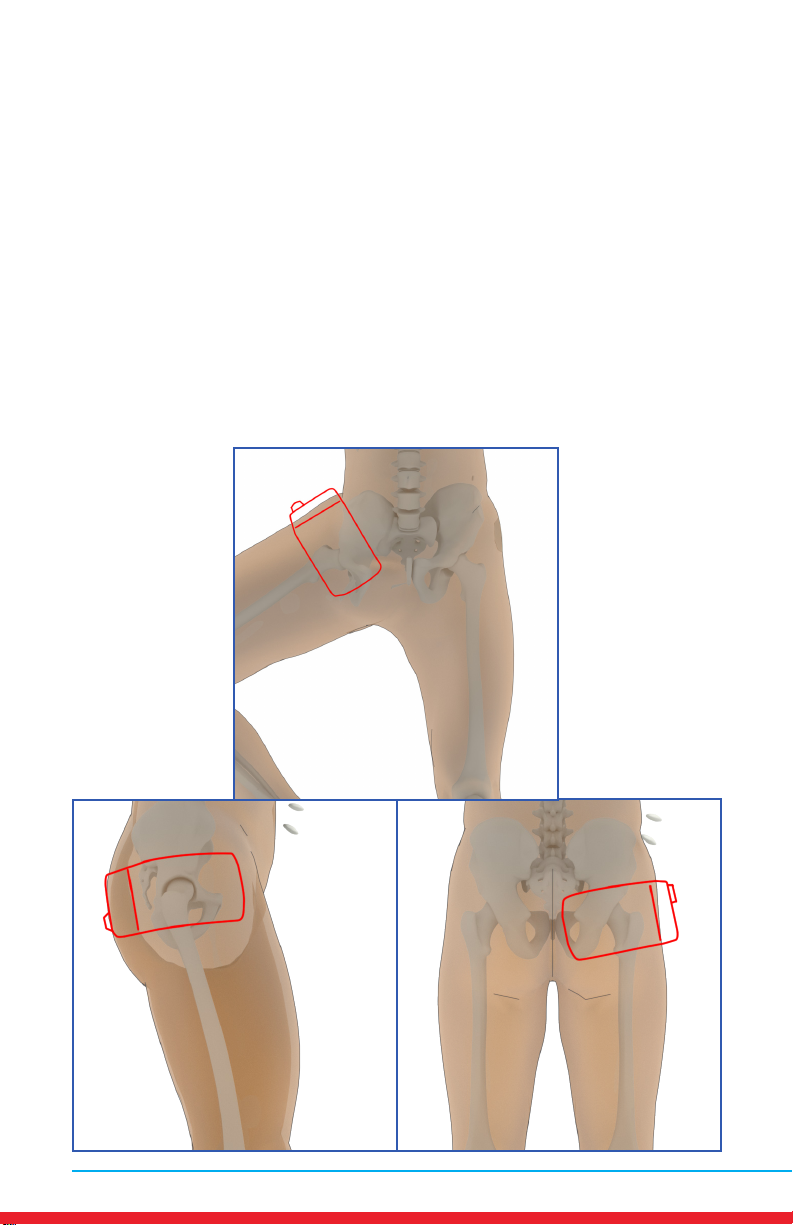
Hip Pain
Placements
• Red outline indicates placement of Array.
• Ensure rm contact of the Array on bare skin.
• Secure the Array using provided straps or small weight
• Once the Array cycles through RED and INFRARED light, re-position to
the next placement and start treatment again.
• Treat around the entire arthritic joint using as many placements as
required (refer to illustrations below).
• Position the Array on muscles adjacent to the arthritic joint if they are
also painful.
Table of contents
Other BIOFLEX Medical Equipment manuals