Contents
4ID: 182463115
5.3.3 Mechanical interactions .................................................................................................... 28
5.3.3.1 Ground and building vibrations......................................................................................... 28
5.3.3.2 Impact noise ..................................................................................................................... 30
5.3.3.3 Acoustic ............................................................................................................................ 30
6 Planning details ............................................................................................................................... 31
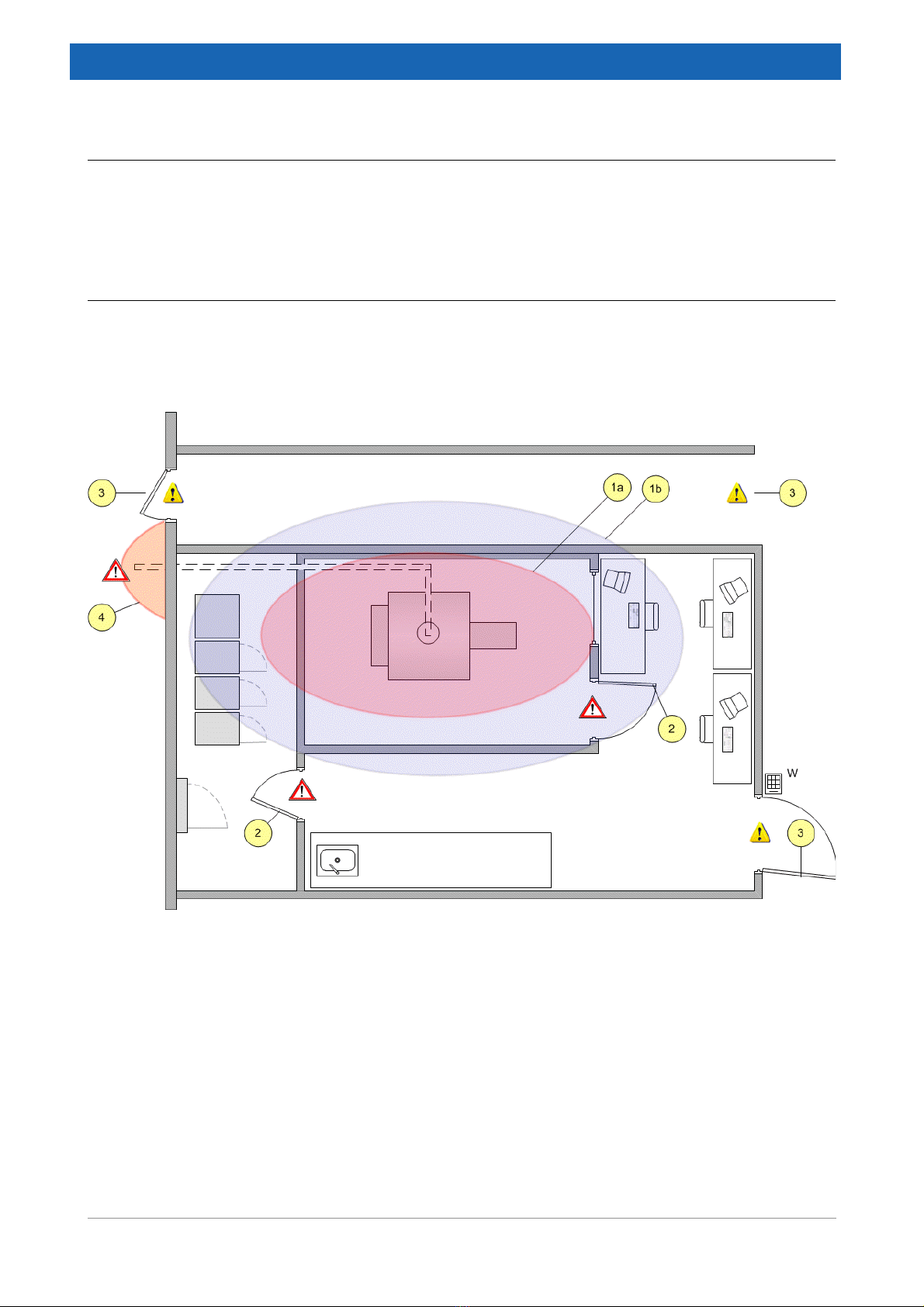
6.1 Measurements and room dimensions .............................................................................. 31
6.1.1 Overview........................................................................................................................... 31
6.1.2 Operating area.................................................................................................................. 32
6.1.3 Magnet area ..................................................................................................................... 32
6.1.4 Technical area .................................................................................................................. 34
6.2 HF shielded room ............................................................................................................. 35
6.2.1 Filter plates ....................................................................................................................... 36
6.2.1.1 Electronic filter plate ......................................................................................................... 40
6.2.1.2 Magnet filter plate ............................................................................................................. 40
6.2.1.3 Filter plate for anesthetic gas exhaust.............................................................................. 40
6.2.1.4 Filter plate for magnet exhaust system............................................................................. 41
6.2.1.5 MRI CryoProbe™ filter plate............................................................................................. 41
6.2.1.6 In-vivo filter plate .............................................................................................................. 41
6.2.1.7 Filter plate for Faraday cage ventilation ........................................................................... 41
6.3 Exhaust system ................................................................................................................ 42
6.3.1 Design criteria of the exhaust system............................................................................... 44
6.3.2 Calculating the exhaust system........................................................................................ 45
6.4 Floor construction ............................................................................................................. 46
6.4.1 Surface loads and weights ............................................................................................... 47
6.4.2 Magnet foundation............................................................................................................ 48
6.4.3 Electrostatic discharge ..................................................................................................... 50
6.4.4 Seismic safety standards.................................................................................................. 50
6.5 Electrical installations ....................................................................................................... 53
6.5.1 Overview........................................................................................................................... 53
6.5.2 Main connection ............................................................................................................... 54
6.5.3 Central potential compensation ........................................................................................ 56
6.5.4 Electrical connections in the magnet room....................................................................... 56
6.5.5 Electrical connections in the operating room.................................................................... 56
6.5.6 Connection and assembly of electrical distributor ............................................................ 57
6.6 Supervision signals........................................................................................................... 57
6.7 Cable lengths and routing................................................................................................. 58
6.7.1 Line lengths for the MRI CryoProbe ................................................................................. 62
6.8 Lighting system................................................................................................................. 63
6.9 Ventilation and air conditioning......................................................................................... 63
6.9.1 Overview........................................................................................................................... 63
6.9.2 Air conditioning systems................................................................................................... 64
6.9.3 Anesthetic gas extraction ................................................................................................. 65
6.10 Cold water supply ............................................................................................................. 66
6.10.1 Connections and installation............................................................................................. 67
6.11 Cryogen liquids................................................................................................................. 70
6.12 Compressed air and gas supply ....................................................................................... 70
6.12.1 MRI CryoProbe™ ............................................................................................................. 70
6.13 Laboratory furnishings ...................................................................................................... 71