This manual assists you with the operation of the C2Dx Model 8002-062-
612, 8002-062-622, and 8002-062-626 Mul-T-Pad® . Read this manual before
operating this product and keep a copy on le. Set methods and procedures
to educate and train your sta on the safe operation of this product.
Intended use
The T/Pump® localized therapy system supplies warm or cold water at
controlled temperatures. Delivery of the water is through the thermal transfer
devices (Mul-T-Pad) for localized temperature therapy. The T/Pump® is for use
in situations where temperature therapy is necessary or desired.
Localized temperature therapy is of particular benet in treating the following:
• Orthopedic such as acute injuries, chronic pain, lower back
pain, muscle spasm, and strains;
• Skin trauma such as abscesses, boils, bruises, burns and contusions;
• Other medical such as chronic s, s, phls,
tends, and IV inltra and symptoms such as inf and
localized pain.
Product descrip�on
The Mul-T-Pad is a lightweight polymer. The colder style connectors provide
the means to connect to the temperature controller. The button design allows
water to ow when the pad is folded to form a customized t.
Expected life
These Mul-T-Pad products have a 30 day single patient use expected life
upon rst use and under normal use conditions.
Contraindica�ons
For heating:
• Applica to a body surface with compromised blood ow (Ischemia,
area under pressure, arterial insuciency).
• Applica to a pant with an increased tendency to bleeding
(aggravates potenal for hemorrhage).
• Applica to a body surface with possibility of malignancy
metabolism is increased and therefore, the growth potenal of the
malignan
• Treatment of hematoma within st 24-48 hours (potenal for re-bleeding
and hemorrhage). Recent sprain or fracture (acute inammatory
response).
• In combina with topical sol whose toxicity may be aected by
the applicaat.
• In combinawith other heat sources.
For cooling:
• Applica to body surface with compromised blood ow (Ischemia,
area under pressure, arterial insuciency
• Applica to body surface with known vascular impairment such as
frostbite, arteriosclerosis or ischemia
• Applica to body surface in people with vity to cold, such
as people with Raynaud’s phenomenon, cold urcaria, cryoglobulinemia,
and paroxysmal cold hemoglobinuria
• Applicato body surface in people with impaired sensa
• In combina with topical sol whose toxicity may be aected by
the applica
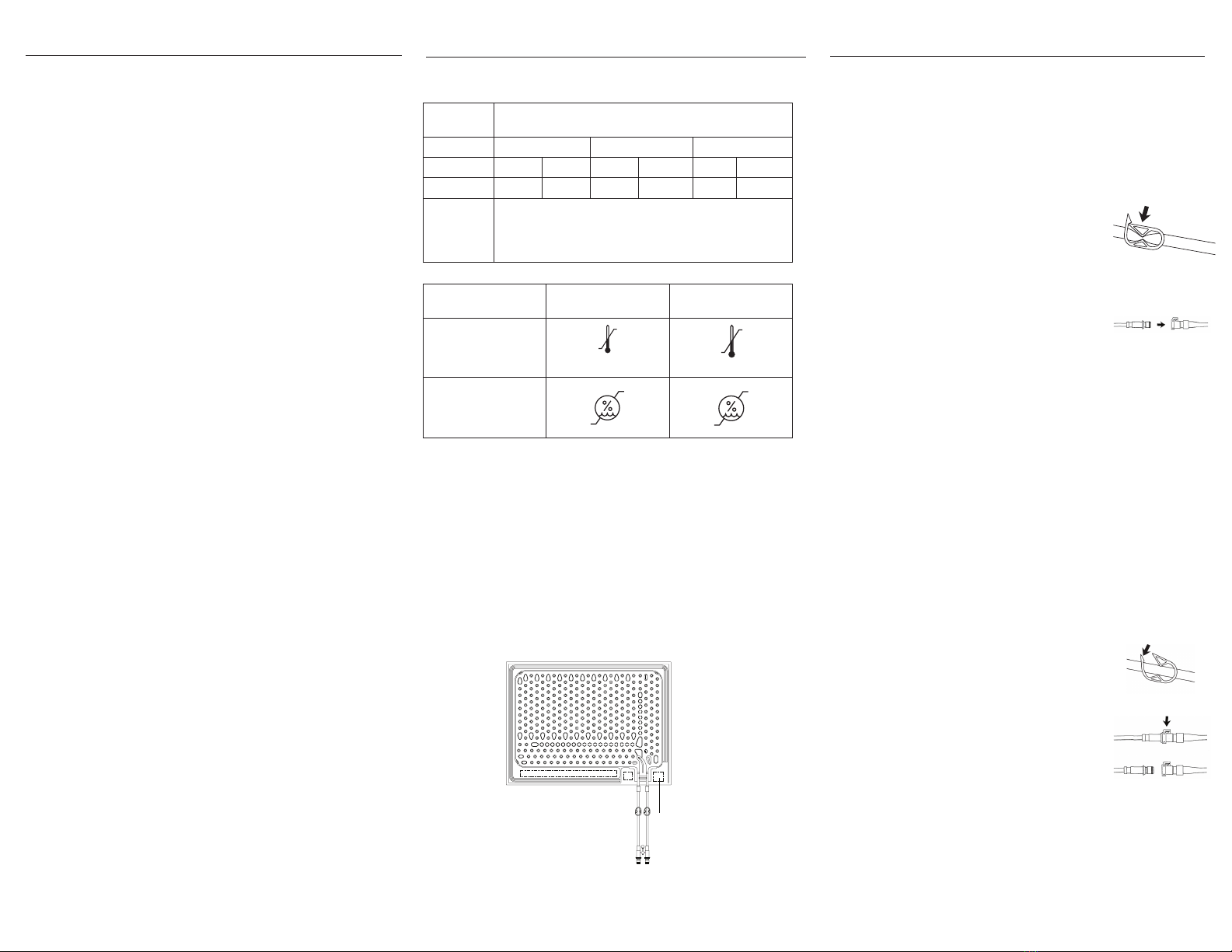
Specifica�ons
Material rayon, ethylene vinyl acetate (EVA), polypropylene,
polyethylene
Model 8002-062-612 8002-062-622 8002-062-626
Length 18 in. 46 cm 22 in. 56 cm 26 in. 66 cm
Width 13 in. 33 cm 15 in. 38 cm 18 in. 46 cm
Compatible
controllers
T/Pump series temperature controllers
Environmental
conditions
Operating Storage and
transportation
Temperature 90 °F
(32.2 °C)
60 °F
(15.6 °C)
120 °F
(48 °C)
-20 °F
(-29 °C)
Relative humidity
30%
75%
10%
95%
C2Dx reserves the right to change specications without notice.
A
Figure 1: Batch lot code
Assessing the paent’s skin condin
Note: Follow your fa skin care protocol. The following instru are
recommendas.
1. Document ial skin assessment.
2. Check for any foreign items such as medical patches, IV’s, sutures.
3. Check for uids such as moisture or gels.
4. Verify the pad size is correct before applying to the pant.
Note: Apply the pad directly to the skin or add minimal layers.
Connec�ng the pad (colder style connectors)
1. Close the pinch clamps on the connector hose and
pad (Figure 2).
2. Insert the male coupling of pad into the female
coupling of hose. Press un you hear a click
(Figure 3).
3. With a light pull, make sure that you locked the
connectors.
4. Open all clamps on the connector hose and the
pad (Figure 4).
5. Insert the power cord into a wall outlet.
6. Press the power buon to turn on the controller. See
the controller instrufor use.
7. Check that the pad lls with water.
Note:
• Iodine based disinfectants will stain the product. Staining does not aect
the pant nor the use of the product.
• Do not use pins or sharp objects with this product.
• If you fold the pad, place the end of the pad that contains the tubing
away from the pant.
Rechecking the pa�ent’s skin condi�on
Check the patient at regular intervals as directed by hospital protocol. Note any
change in the skin integrity that relates to:
• Excessive moisture - dry the skin surface by wiping away the moisture
• Color of the epidermis
• Skin texture
• Pant’s skiacceptable to connue therapy
Check the pad
Check the pad at regular intervals as directed by hospital protocol.
• p
• water status
• dry surface
• leaks
• cracks
Defibrilla�on considera�ons
1. Remove the pad to expose pant’s chest.
2. Remove excess moisture.
Disconnec�ng the pad from the hoses
1. Power ontroller.
2. Close pad clamps (Figure 2).
3. Press down on the thumb tab of the female coupling. Pull the male
coupling out to disconnect (Figure 5).
4. Discard pad afacility protocol.
Note: Do not discard the controller connector hose after use, save for next
use.
Figure 5: Disconnect
Figure 2: Close
Figure 4: Open
Figure 3: Connect
Opera�onIntroduc�onIntroduc�on
Contact informa�on
Contact C2Dx Customer Service at 1-888-902-2239.
C2Dx, Inc.
555 E. Eliza St. | Ste. A
Schoolcraft, MI 49087 USA
Have the Lot batch code of your C2Dx product available when calling
C2Dx Customer Service or Technical Support. Include the Lot batch code
in all written communication.