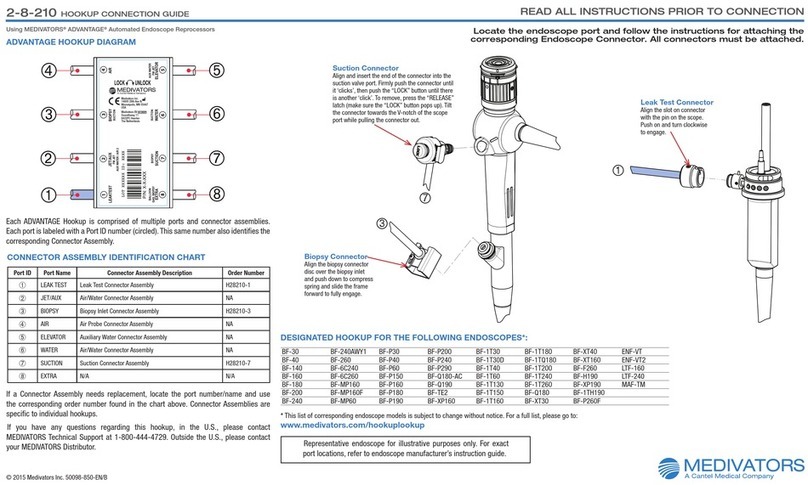
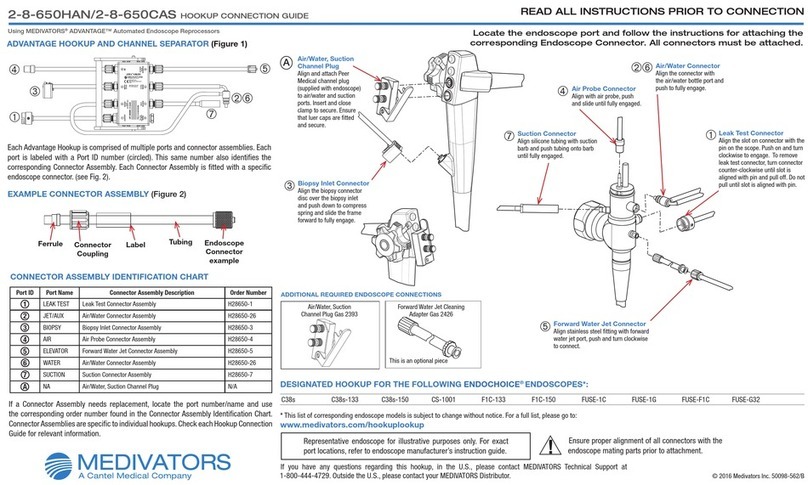
5
WARNINGS
A. To reduce risk of electrical shock, do not remove cover. Refer servicing to qualied service personnel.
B. To avoid the risk of electric shock, this equipment must only be connected to a supply mains with protec-
tive earth.
C. The ENDO STRATUS® CO
2
insufator is not suitable for use in the presence of a ammable anesthetic
mixture with oxygen.
D. The ENDO STRATUS CO
2
insufator shall be sold only by prescription for use by physicians/clinicians who
are trained and qualied.
E. Only qualied medical personnel in an acceptable medical facility should operate the ENDO STRATUS CO
2
insufator.
F. The ENDO STRATUS CO
2
insufator should be connected to a properly grounded receptacle marked
“Hospital Grade” or “Hospital Only,” otherwise grounding reliability cannot be achieved.
G. Extreme precaution must be taken when handling liquids around electrical equipment. DO NOT operate
the
ENDO STRATUS CO
2
insufator if liquid has been spilled on the unit.
H. Never place or stack the ENDO STRATUS CO
2
insufator on other electrical equipment other than the
ENDO STRATUS irrigation pump. Electromagnetic or other interference may occur between the ENDO
STRATUS CO
2
insufator and other electronic devices. The equipment or system should be observed to
verify normal operation in the conguration in which it will be used.
I. The ENDO STRATUS CO
2
insufator should only be used in conjunction with other equipment whose
safety against leakage currents has been established.
J. The instructions contained in the operating manuals of any equipment to be used in conjunction with the
ENDO STRATUS CO
2
insufator must be followed to avoid any possible hazard from incompatibility.
K. The instructions for use described in this manual MUST be followed. Otherwise, compromised safety,
malfunction, injury to the operator and/or patient, or costly damage to the unit and other equipment may
occur.
L. The ENDO STRATUS CO
2
insufator must be connected to an appropriate power source when loss of
power source would result in an unacceptable risk.
CAUTION
A. If emergency or abnormal function occurs, immediately turn off the power to the unit.
B. Use only medical USP medical grade CO
2
from a “D” or “E” size CO
2
tank.
C. Read and understand all warnings that come with your commercially available “D” or “E” sized CO
2
tanks.
D. Field-servicing of the ENDO STRATUS CO
2
insufator is limited to the replacement of the power cords,
high pressure hoses, yoke adapters, water bottle holder and heater assemblies, and fuses.
E. Remove power from the device before initiating any eld servicing of the replacement parts.
F. There are no user serviceable parts inside this unit. Repairs to the ENDO STRATUS CO
2
insufator should
only be performed by qualied service personnel. No user modication of this equipment is allowed.
G. When the low pressure warning indicator illuminates, exchange the CO
2
tank as soon as possible to avoid
loss of function.
H. Always keep a spare full tank of CO
2
nearby for quick access.
I. Always keep the CO
2
tank in an upright position to avoid uid entering the unit.
J. Do not use the device if the enclosure is damaged or enclosure integrity has been compromised.
K. Do not attempt to operate the device before reading and understanding all sections of this manual.
L. Medical electrical equipment needs special precautions regarding EMC and needs to be installed and put
into service according to the EMC information provided in section X.
M. Portable and mobile radio frequency (RF) communications equipment can affect medical electrical
equipment. Do not expose the device to sources of electromagnetic interference such as CT equipment,
diathermy equipment, cellular phones, RFID tags and metal detectors.
INTERNATIONAL_ IFU-52 (CO2 INSUFLATOR PUMP) REV H .indd 5 12/11/2015 9:31:55 AM