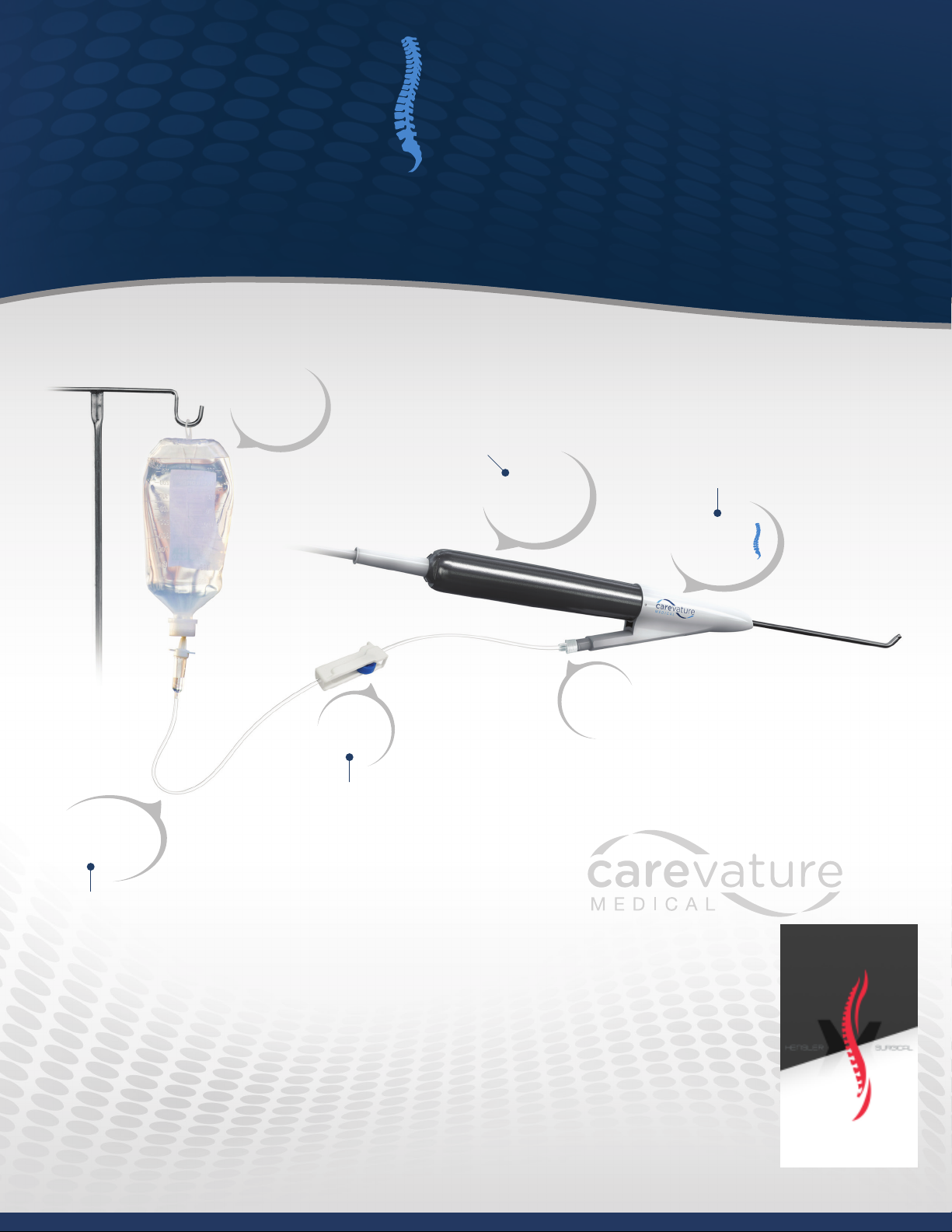
INDICATIONS FOR USE
The Carevature Dreal™ is intended to be used with high-speed compatible electric and pneumatic motors.
When used with these motors, it is intended to cut bone by drilling, reaming, decorticating, shaping,
dissecting, shaving and smoothing for neurosurgical and spinal applications. Specic applications include
laminectomy/laminotomy and craniotomy/craniectomy/ skull base cuts < 1cm3.
General Operating Guidelines:
The Carevature Dreal™ is intended to be used with electric and pneumatic motors and acts as an interface
between the high-speed motors and the cutting accessories.
This symbol is used to alert the reader to important safety and precautionary information.
When displayed on the actual device or packaging, it refers the user to the instruction manual.
CAUTION
Federal (USA) law restricts this device to sale and use by, or on the order of a physician.
The words Warning, Caution and Note carry special meanings and should be carefully reviewed.
Important Safety Information
WARNING
• You must read carefully the entire instructions and warnings listed below prior to use.
• DO NOT use the DrealTM after the “Use By” date on the package label.
• DO NOT use the DrealTM with any motor other than one having the mentioned specications (See Motor
Selection and Handling section below)
• DO NOT use the DrealTM other than as a single patient, single use device. Reuse, reprocessing, or
re-sterilization of the device is strictly prohibited as reuse of the device can compromise the DrealTM
performance characteristics.
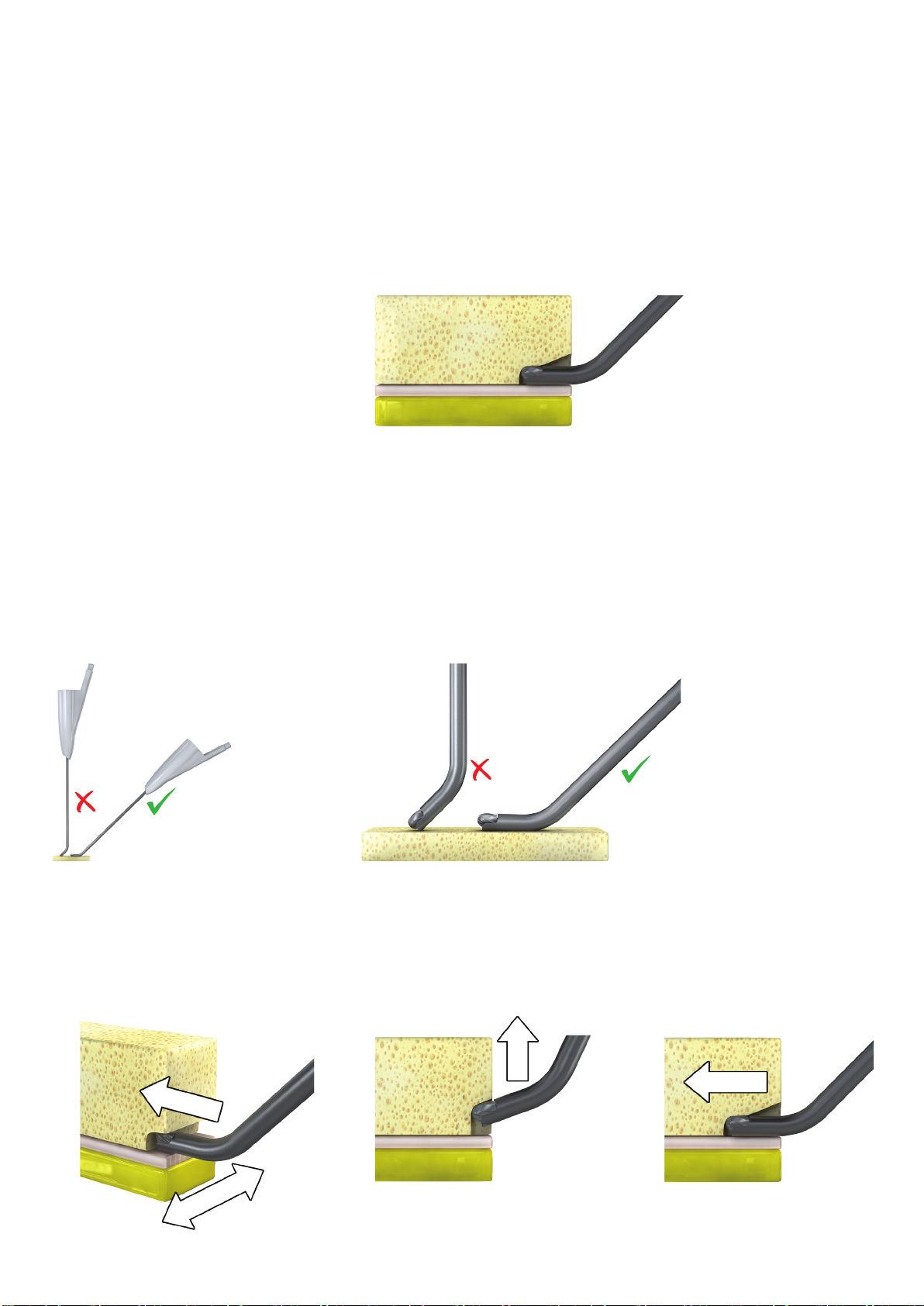
• DO NOT use the DrealTM without active irrigation to prevent tissues damage.
• DO NOT use the DrealTM without eyewear protection.
• DO NOT use the DrealTM if, after inspecting the DrealTM and its package, there is any overt damage. In
the event of damage to the sterile packaging, retain the package with the contents and notify your local
authorized representative.
• DO NOT use the DrealTM if damage is apparent or if the device does not function normally. The DrealTM
must be inspected prior to each use.
• DO NOT use excessive force which may bend the device’s shaft or cutter. This may cause the tool to
deform and may result in malfunction, injury to the patient, operator and/or operating room staff.
• The DrealTM should be used only by medical professionals familiar with powered surgical instrumentation;
this package insert is designed to assist in using the Dreal™ device and it is not a reference to surgical
technique. For information on surgical technique, please consult the medical literature.
• It is not recommended to use the Dreal™ continuously for more than 10 minutes.
• For any malfunctioning or damaged packages or devices, please contact your local authorized
representative
Potential Complications
• As in any rotatable devices, if not used properly or in case of inadvertent dislocation of the cutting tip
when active, an injury to the patient may occur.
Contraindications
• There are no known contraindications for devices with these indications.
2