SYSTEM USE
2.1 POSITION PATIENT ON BED
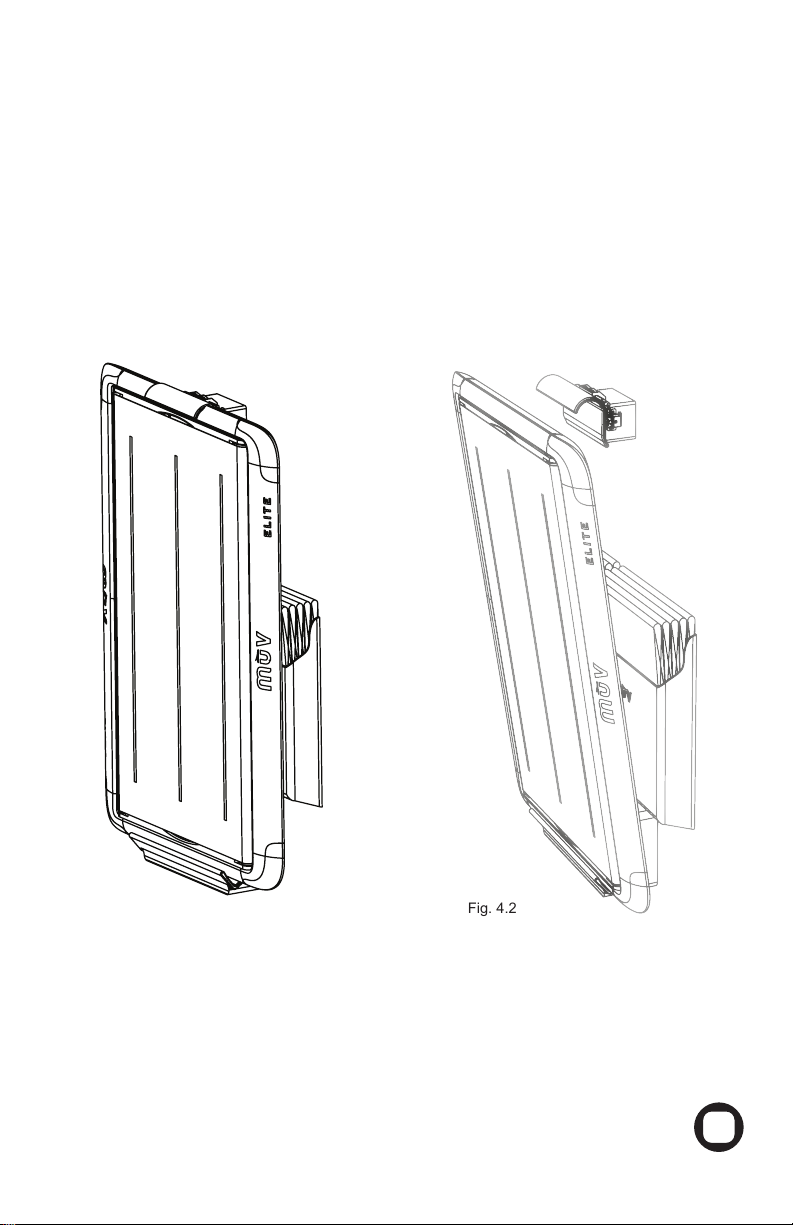
After loading the MUV™ELITE with the Multi-Purpose PAD, roll the patient to a lateral
recumbent position facing away from you (Fig 8.1). Manage and clear cables, tubes, and
monitoring equipment before placing the MUV™ELITE.
2.2 PLACE MUV™ELITE
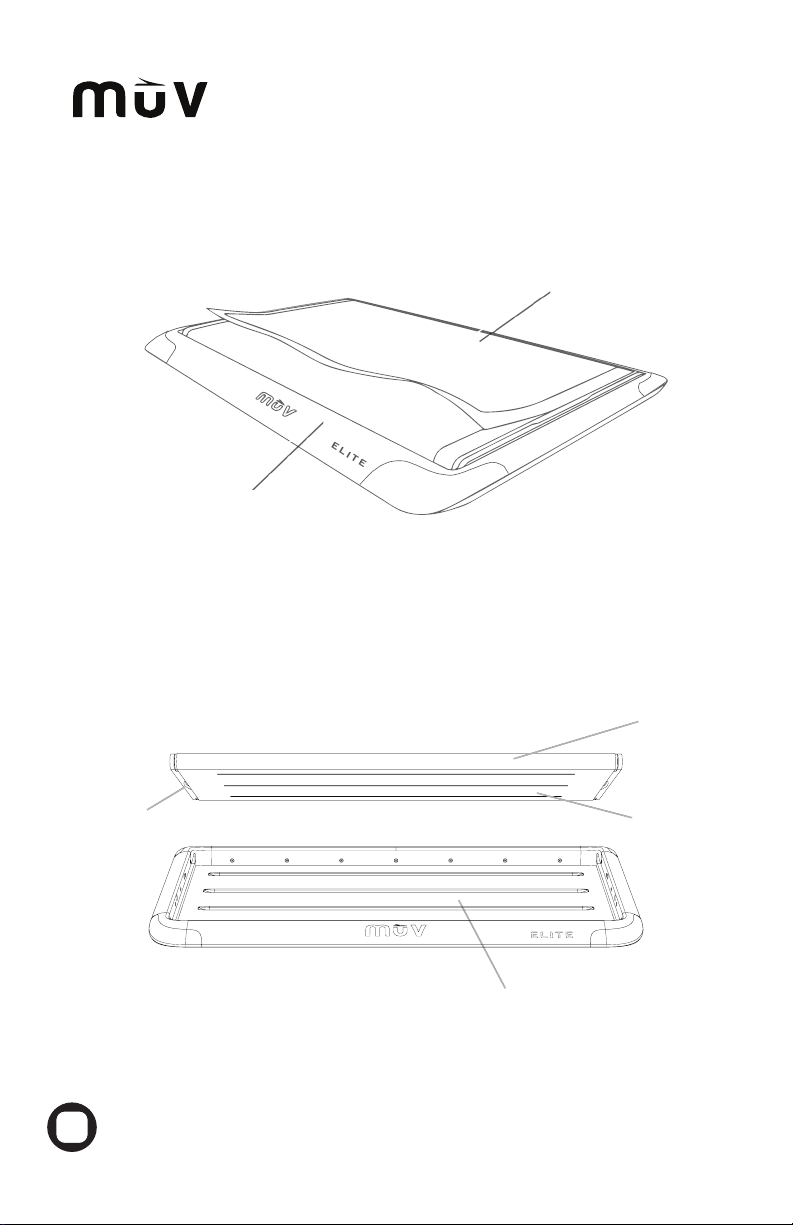
Place the leading edge of the MUV™ELITE under approximately 2/3 of the patient’s back
(Fig. 8.1). Each leading edge has the MUV™logo on it. The MUV™ELITE serves as a bridge
between the two lateral surfaces.
2.3 POSITION PATIENT ON BOARD
Gently roll the patient’s back onto the MUV™ELITE (Fig. 8.2).
2.4 TRANSFER STAFF TEAM MEMBER ROLES
Team member 1 engages the MUV™ELITE by pushing the patient across the MUV™ELITE.
Team member 2 guides the MP PAD to the second bed in a continuous motion (Fig. 8.2).
Allow the MUV™ELITE to do the work of the transfer. Team member 3 guides the feet, while
a fourth team member (not pictured) manages the patient’s head and airway.
2.5 COMPLETE THE TRANSFER
The patient completes the transfer when he or she is on the second lateral surface. The MP
PAD stays beneath the patient. No further patient manipulation should be required.
2.6 REMOVE MULTI-PURPOSE PAD FROM MUV™ELITE
Detach the MP PAD by pulling the MUV™ELITE away from the patient. (In the event the
patient is still partially on the MUV™ELITE following the transfer, simply tip the MUV™ELITE
and pull from under the patient. Always be aware of abrasions or skin integrity issues
related to any patient transfer.) When soiled or medically indicated, or as otherwise dictated
by your facility’s policies, discard the MP PAD in the appropriate receptacle as medical
waste.
7