6
Device Overview
Quick-20m Overview
EEG is the measurement — through the use of sensors and
ampliers — of scalp surface electrical potentials arising
from activity in the cortex.
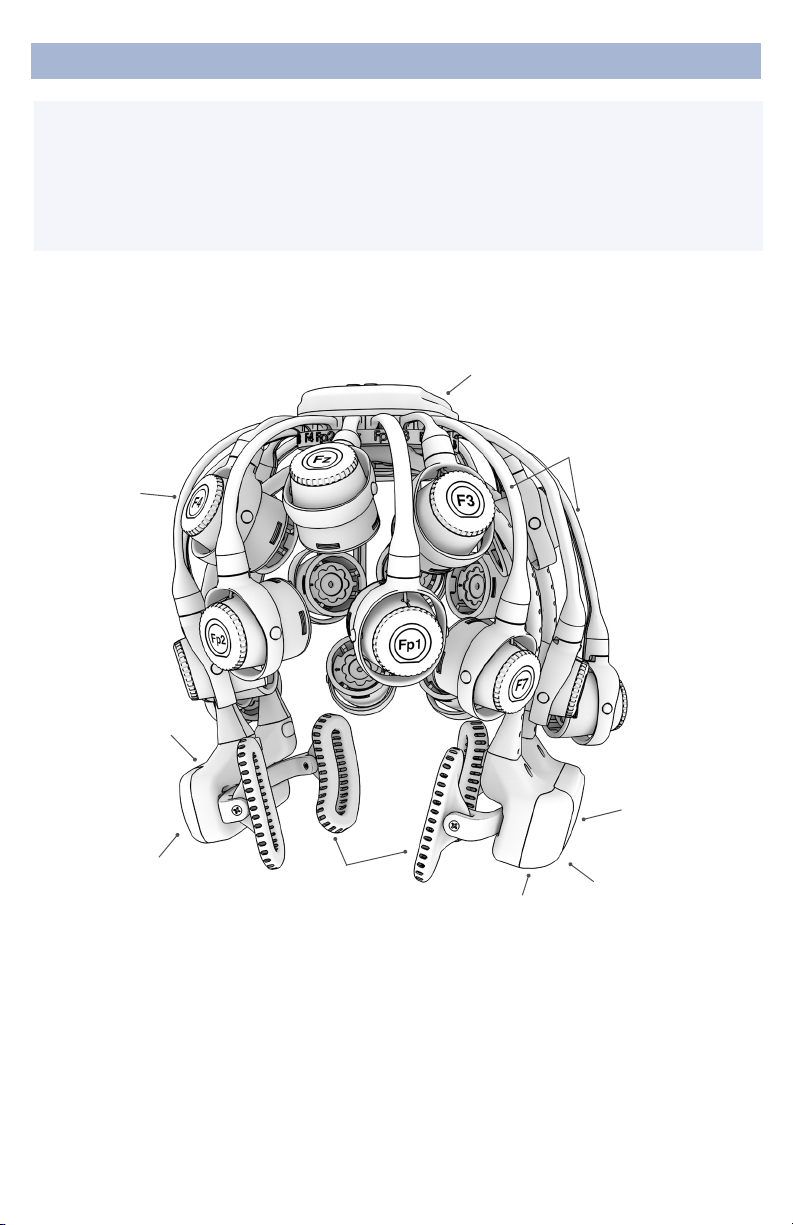
The Quick-20m follows the international 10-20 system
standardizing electrode placements across the scalp. The
10-20 system derives its name from measurements of 10%
and 20% of the distance between landmarks on the head,
specically, the depression above the bridge of the nose
(nasion), the bump at the back of the head (inion), and the
depressions in front of each ear (preauricular points).
Sensors are labeled with a combination of letters and
numbers indicating their respective locations, with even
numbers on the right and odd numbers on the left side of
the head. Midline locations are marked with a “z” for “zero.”
For instance, Fz sits on the frontal midline, while F3 sits left
of and F4 sits right of Fz.
The Quick-20m is sized to t heads with a circumference
between 52 and 62 cm as measured about the thickest
part of the skull.
The Quick-20m meets the
mechanical, electrical, and
sensor needs required to
make an eective dry EEG
system.
Conventional wet systems
rely on electrolytic gels
to penetrate hair, contact
the skin, and provide a
conductive path. The gel
serves as a buer lling in
gaps between the sensor and
skin.
No conductive gel is used in a
dry system. The benets are
obvious: faster set-up, and no
after-use clean-up required.
Yet, dry systems are prone
to several challenges. First,
the sensor must be designed
to directly touch the scalp or
skin, even through thick hair.
Second, the sensor must
remain securely in place to
minimize artifacts and noise.
Finally, the electronics must
tolerate impedances up to
200 times higher than wet
systems — while rejecting
noise and interference. A
high-end dry solution — like
the Quick-20m — balances
sensors, mechanics and
electronics achieving virtually
the same signal quality as a
traditional wet cap for most
EEG applications.