Copyright © 2019, Condor InstrumentsLtda. 5
Professor TúlioAscarelli St.,290 - Vila Madalena, São Paulo, SP, Brazil - Cep:05449-020
Introduction
Before using thedevice,read the usermanualas a whole.
Indications of Use
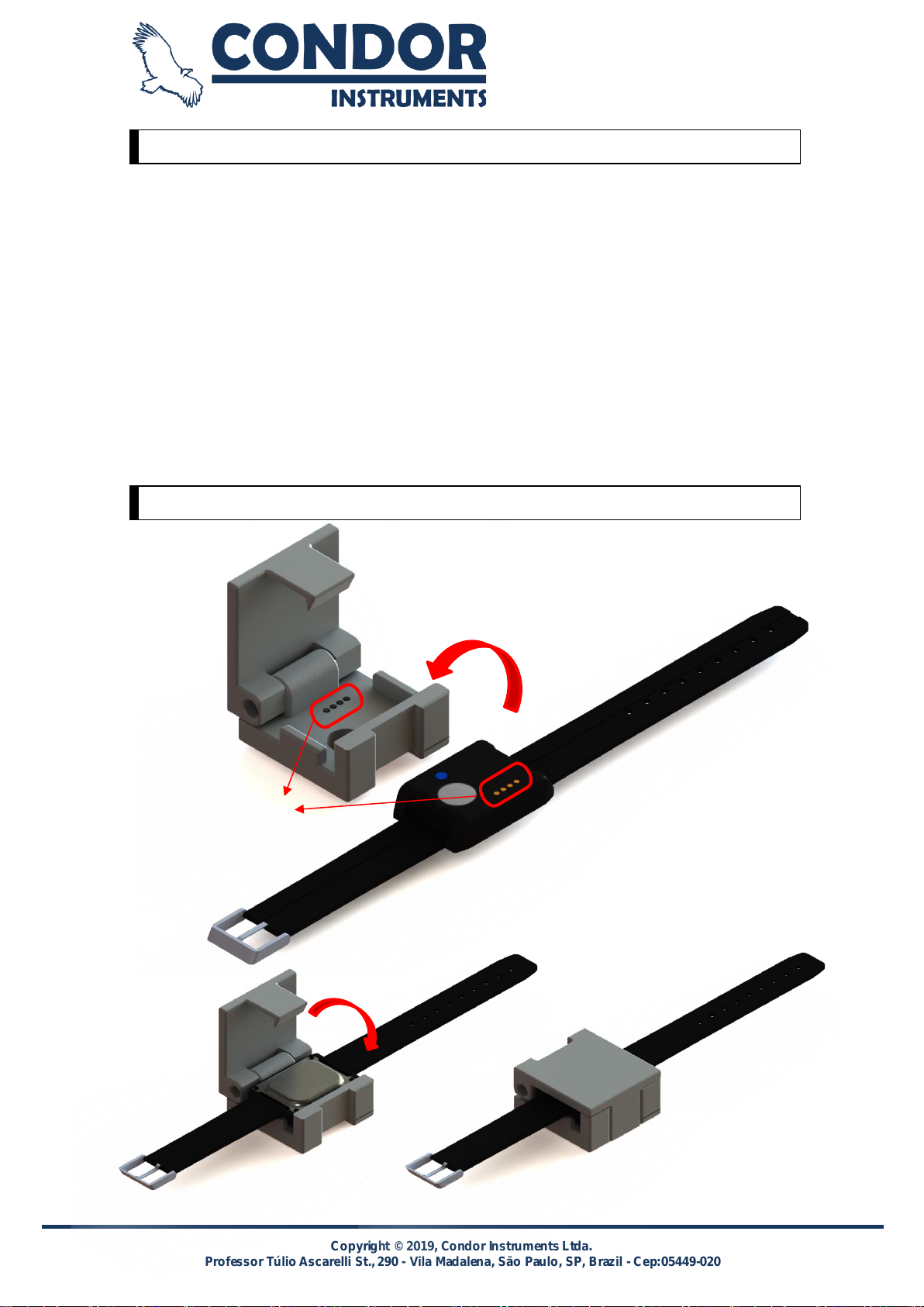
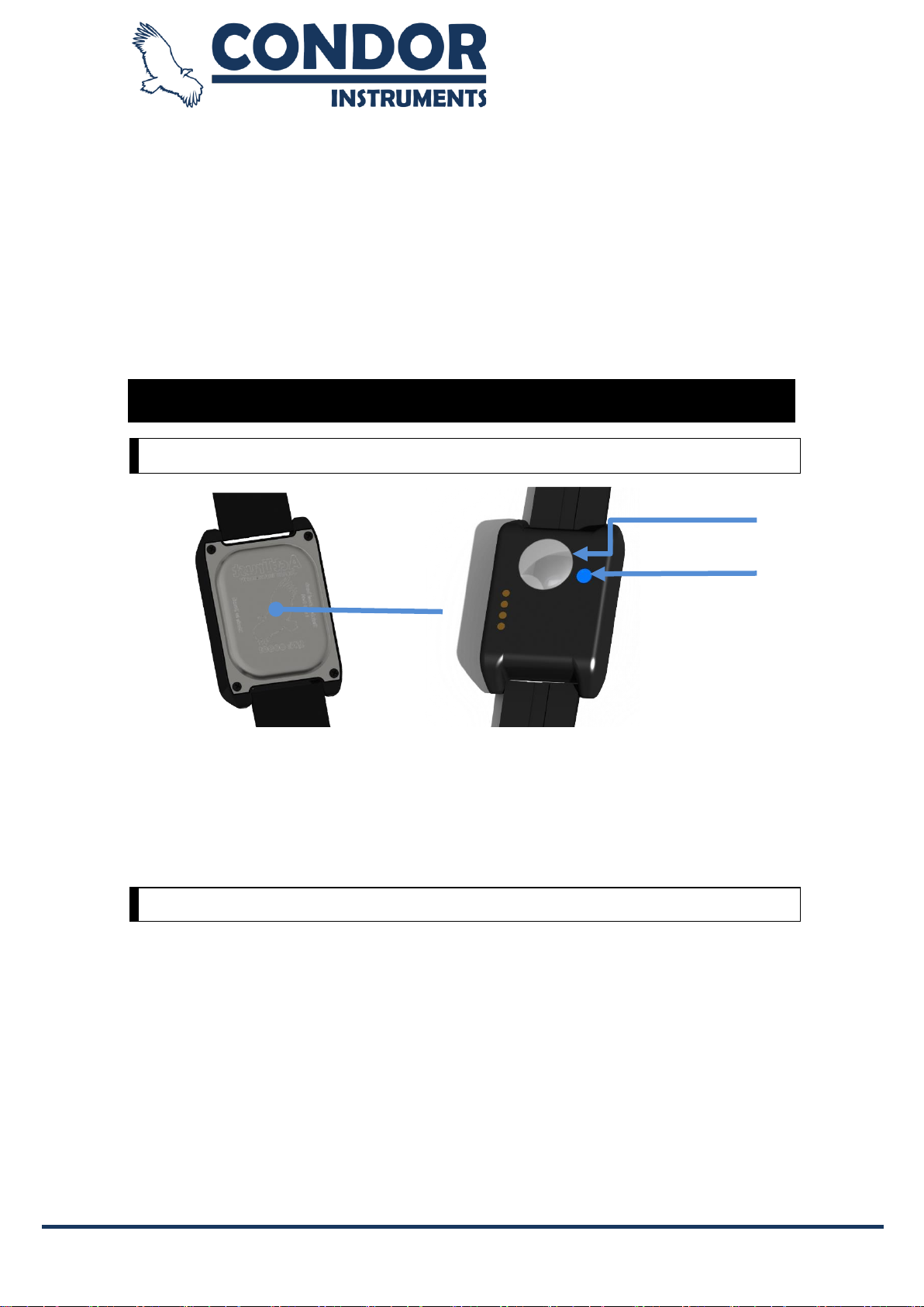
The ActTrust is an ultra-compact,lightweight, wrist-worn activity, temperature and ambientlight monitor that can
be used to analyze circadian rhythms, automatically collect and store data for sleep parameters, and assess
activity in any instance where quantifiable analysis of physical motion is desirable. The device is intended to
monitor limbor body movements duringdailylivingandsleep.
Contraindications
Thereareno contraindicationsto theuseofthedevice.
Adverse Effects
Patients should informyourdoctor and discontinueuse immediately iftheyexperience itchingor irritationin the
contactareaofthedevicewiththeskin.
Maintenance
Sterilizationis notrequiredfornaypartoftheproduct.
PRECAUTIONS
Inspections and repairs should only be performed by an authorized agent. Under no circumstances should you
attemptto openor personallycarryout repairsor maintenance of thedevice.
This product must be inspected by a Condor authorized service center five years from thedate of manufacture,
before that, the device is intended to provide a safe and reliable operation provided that it is operated and
maintained in compliance with the instructions provided by Condor. Thedetails concerning the Condor warranty
applicable areprovided with thedevice fromits original purchase.Ofcourse,like all electrical devices, becareful
and request inspectionataCondorauthorizedservicecenterif any malfunctions aredetects.
Warnings and General Precautions
Warnings
A warning to thepossibilityofpersonal injury.
•Beforeusingthedevice,read theentiremanual.
•The device and its accessoriesmustbe used onlyfor thepurposefor which theyare intended.
•Only useaccessoriesand partsthatareoriginalandapprovedby Condor.
Additional equipment connected to medical electrical equipment must comply with the respective IEC or ISO
standards (p. Ex., IEC 60950 relating to data processing equipment). In addition, all configurations must meet
the requirements for medical electrical systems (see IEC 60601-1-1or clause 16of the 3rd Ed. Of IEC 60601-1,
respectively). Anyone who connects additional equipment to medical electrical equipment configures a medical
system and is therefore responsible for the system to comply with the requirements for medical electrical
systems. Itcalled attention to the fact that local laws take precedenceover the above requirements. If in doubt,
contactyourlocalrepresentativeorservicedepartment.
Training and Qualification
Any operatormusthaveread themanualandhavethe computer skills necessaryto configurethedevice
The analysisshouldonlybemadeby peoplewhoarequalifiedto makethem