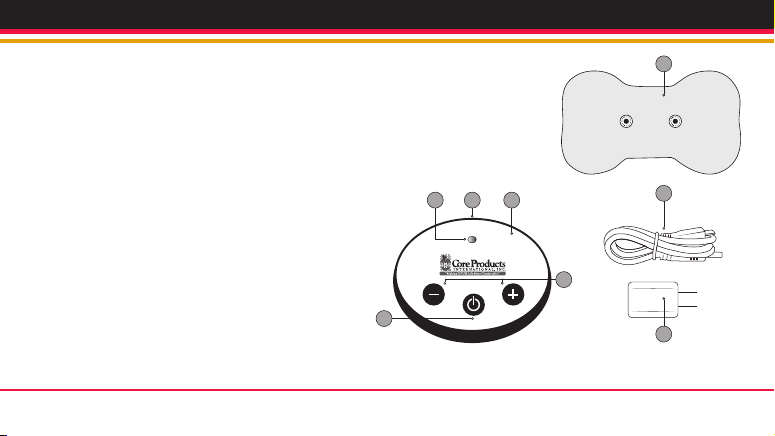
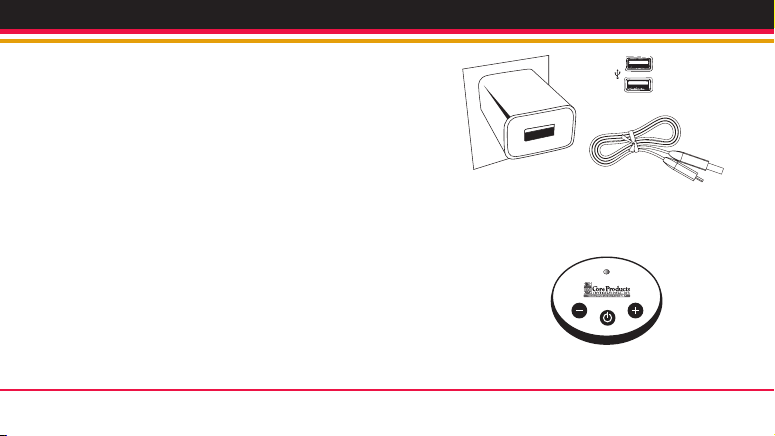
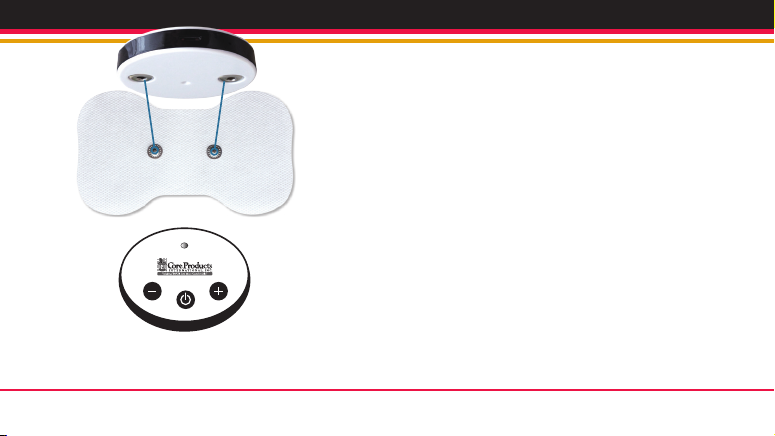
67
Contraindications
1. DO NOT use this device if you have a cardiac pacemaker, implanted defibrillator, or other implanted
metallic or electronic devices. Such use could cause electric shock, burns, electrical interference, or
death.
2. DO NOT use this device if you have any of the following medical conditions:
• Undiagnosed pain syndromes
• Have been diagnosed with cancer
• Are pregnant
• Have suered acute trauma or surgical procedure in the past six months
• Have cardiac problems or cardiac disease
• Have epilepsy or other seizure disorder
• Have painful and/or atrophied muscles
• Have abdominal or inguinal hernia
• Have limited range of motion in skeletal joints
• Have blood circulatory problems
3. DO NOT use this device on muscles that are: atrophied, painful, suer spasms, on a limb with painful or
otherwise aicted joints.
Precautions
1. TENS is not eective for pain of central origin, including headache.
2. TENS is not a substitute for pain medications and other pain management therapies.
3. TENS devices have no curative value.
4. TENS is a symptomatic treatment and, as such, suppresses the sensation of pain that would otherwise
serve as a protective mechanism.
5. Eectiveness is highly dependent upon patient selection by a practitioner qualified in the management
of pain patients.
6. The long-term eects of electrical stimulation are unknown.
7. You may experience skin irritation or hypersensitivity due to the electrical stimulation or electrical
conductive medium (gel).
8. Use this device only with electrodes and accessories recommended by the manufacturer.
9. The Pain Remedy is not intended for the application of any medical condition or disease nor is it
intended for physiotherapy or muscle rehabilitation. It is contraindicated for use on any muscle that is
injured or diseased.