Study Portal Instructions for Use Ι As of 2023-01-25 Ι Revision status: 3
1. TABLE OF CONTENTS
1. TABLE OF CONTENTS ..................................................................................................................................... 3
2. INTRODUCTION ............................................................................................................................................ 4
3. SAFETY INSTRUCTIONS ................................................................................................................................. 5
3.1. WARNINGS ........................................................................................................................................... 5
3.2. CAUTIONS ............................................................................................................................................. 6
3.3. NOTES ................................................................................................................................................... 6
3.4. Indications for Use ................................................................................................................................ 7
3.5. Clinical Benefit ...................................................................................................................................... 7
4. SYMBOLS ...................................................................................................................................................... 8
5. STUDY PORTAL ............................................................................................................................................. 9
5.1. Sign-Up ................................................................................................................................................. 9
5.2. Sign-In ................................................................................................................................................ 10
5.3. Studies Dashboard .............................................................................................................................. 11
5.4. Add Study ........................................................................................................................................... 11
5.5. Delete Study ....................................................................................................................................... 11
5.6. Assign Subjects ................................................................................................................................... 12
5.7. Subjects Overview .............................................................................................................................. 13
5.8. Vouchers ............................................................................................................................................. 15
5.9. Un-assign Subject ................................................................................................................................ 16
5.10. Subject Portfolio ............................................................................................................................. 16
6. STUDY SETTINGS ......................................................................................................................................... 18
6.1. Selection Bracelet ............................................................................................................................... 18
6.2. Vital Parameters ................................................................................................................................. 18
6.3. Raw Data ............................................................................................................................................ 19
6.4. Battery Life ......................................................................................................................................... 20
6.5. Corsano App Settings .......................................................................................................................... 20
7. EXPORT DATA ............................................................................................................................................. 22
7.1. For a Study .......................................................................................................................................... 22
7.2. For Individual Subjects ........................................................................................................................ 24
8. DATA .......................................................................................................................................................... 25
8.1. Summaries .......................................................................................................................................... 25
8.2. Raw Data ............................................................................................................................................ 28
8.3. Data Processing Tools ......................................................................................................................... 29
9. API .............................................................................................................................................................. 30
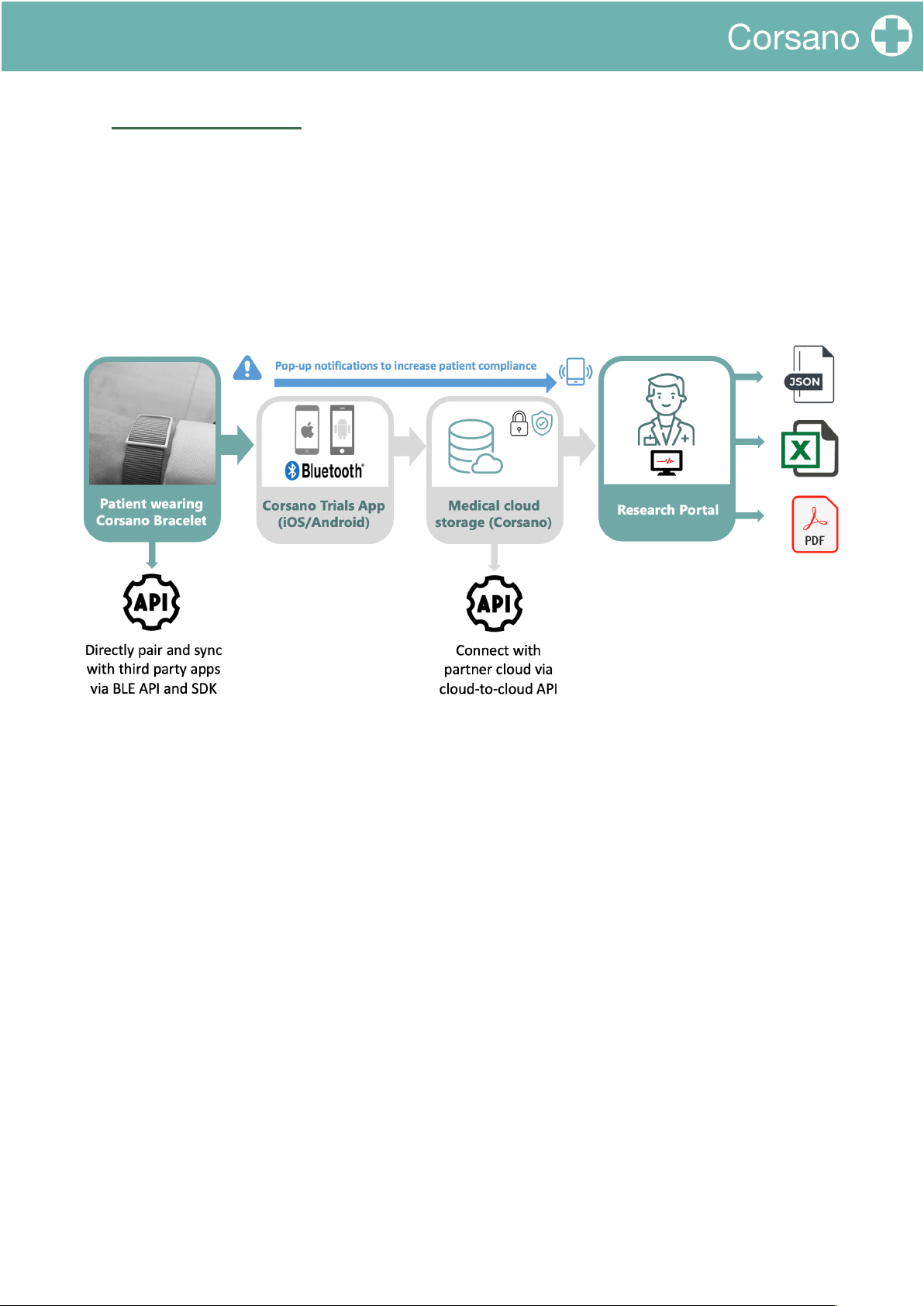
9.1. Corsano Cloud System ........................................................................................................................ 30
9.2. Users Cloud ......................................................................................................................................... 30
9.3. Health Cloud ....................................................................................................................................... 32
9.4. Developer Knowledge Base ................................................................................................................. 34
10. CYBERSECURITY ...................................................................................................................................... 35
10.1. Information Security Management System ..................................................................................... 35
10.2. About password policies, password expiration and auto-logout ...................................................... 35
10.3. About periodical software updates and patches ............................................................................. 36
10.4. Dealing with a lost or stolen Corsano Bracelet ................................................................................ 36
10.5. General Guidelines for Security ....................................................................................................... 36
11. WARRANTY ............................................................................................................................................. 37
12. CORSANO CONTACT INFORMATION ....................................................................................................... 38