4
I. INTRODUCTION
1.0 INTRODUCTION TO RADIOSURGERY®
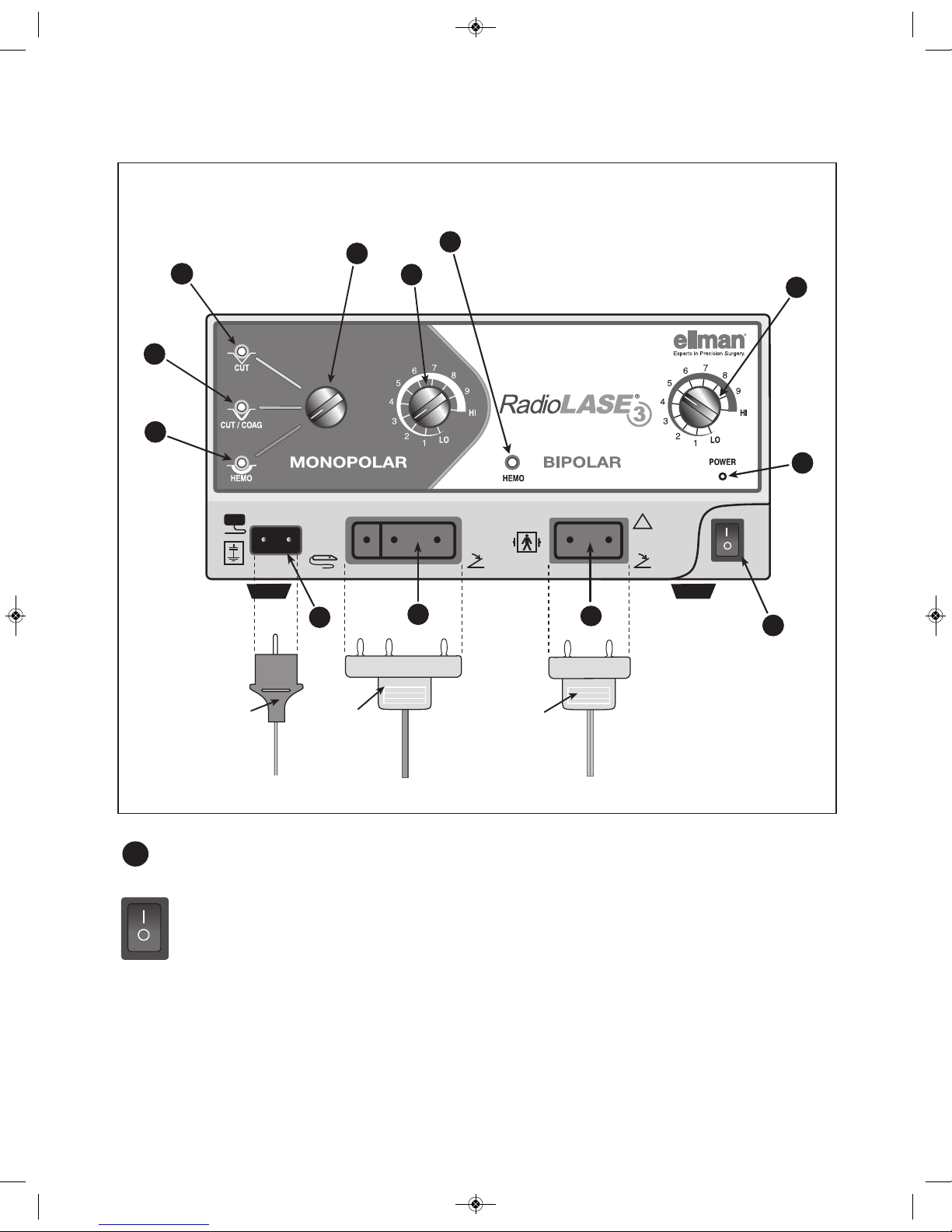
Radiosurgery®with the Radiolase®3 igh Frequency Radio surgical Generator (Generator) is an
excellent method of cutting and coagulating soft tissue. With four waveforms (3 monopolar and 1
bipolar) and a wide selection of electrode tips, the user can easily configure the system for a wide array
of applications.
1.1 INTENDED USE
The intended use for the Radiolase®3 Generator is:
1.1.1 Monopolar Cutting: Skin and Mucosal Incisions, Biopsy, Cysts, Abscesses, Tumors,
Cosmetic Repairs, Development of Skin Flaps, Skin Tags, Nevi, Keratosis, Oculoplastic
Procedures, Blepharoplasty, Aponeurotic Repair, Levator Resection.
1.1.2 Monopolar Blended Cutting & Coagulation: Skin Tags, Papilloma, Keloids, Keratosis,
Verrucae, Basal Cell Carcinoma, Nevi, Fistulas, Epithelioma, Cosmetic Repair, Cysts,
Abscesses, Development of Skin Flaps, Oculoplastic Procedures.
1.1.3 Monopolar emostasis: Control of Bleeding, Epilation, Telangiectasia.
1.1.4 Bipolar emostasis: Pinpoint emostasis in any field (Wet or Dry).
Note: The Radiolase®3 Generator is not indicated for non-ablative treatment of wrinkles.
Furthermore, the Radiolase®3 Generator is neither designed to provide the required power
levels, nor withstand the demands of lengthy non-ablative wrinkle treatment procedures.
The Radiolase®3 Generator will overheat and possibly fail if used for non-ablative treatment
of wrinkles. Such failures will not be covered by warranty.
II. SAFET
Improper use of this, or any high frequency surgical instrument, can result in a patient injury.
Read and become familiar with all instructions and directions before using this product.
2.0 WARNINGS
2.0.1 igh frequency surgical equipment should be used only by persons having adequate
training and familiarity with high frequency surgical equipment and related techniques.
A thorough understanding of the use of high frequency surgical equipment is essential to
avoid shock and burn hazards to both patient and medical personnel and damage to the
device or other medical equipment. Consult medical literature relative to related
techniques, complications, and hazards prior to use of high frequency surgical equipment.
2.0.2 Never increase the power settings without first checking both the active electrode and the
Neutral Pad.
2.0.3 Non-conductive Corneal Eye Shields (Plastic) must be used for any radiofrequency
surgical procedure involving the eyelid and the immediate surrounding areas.
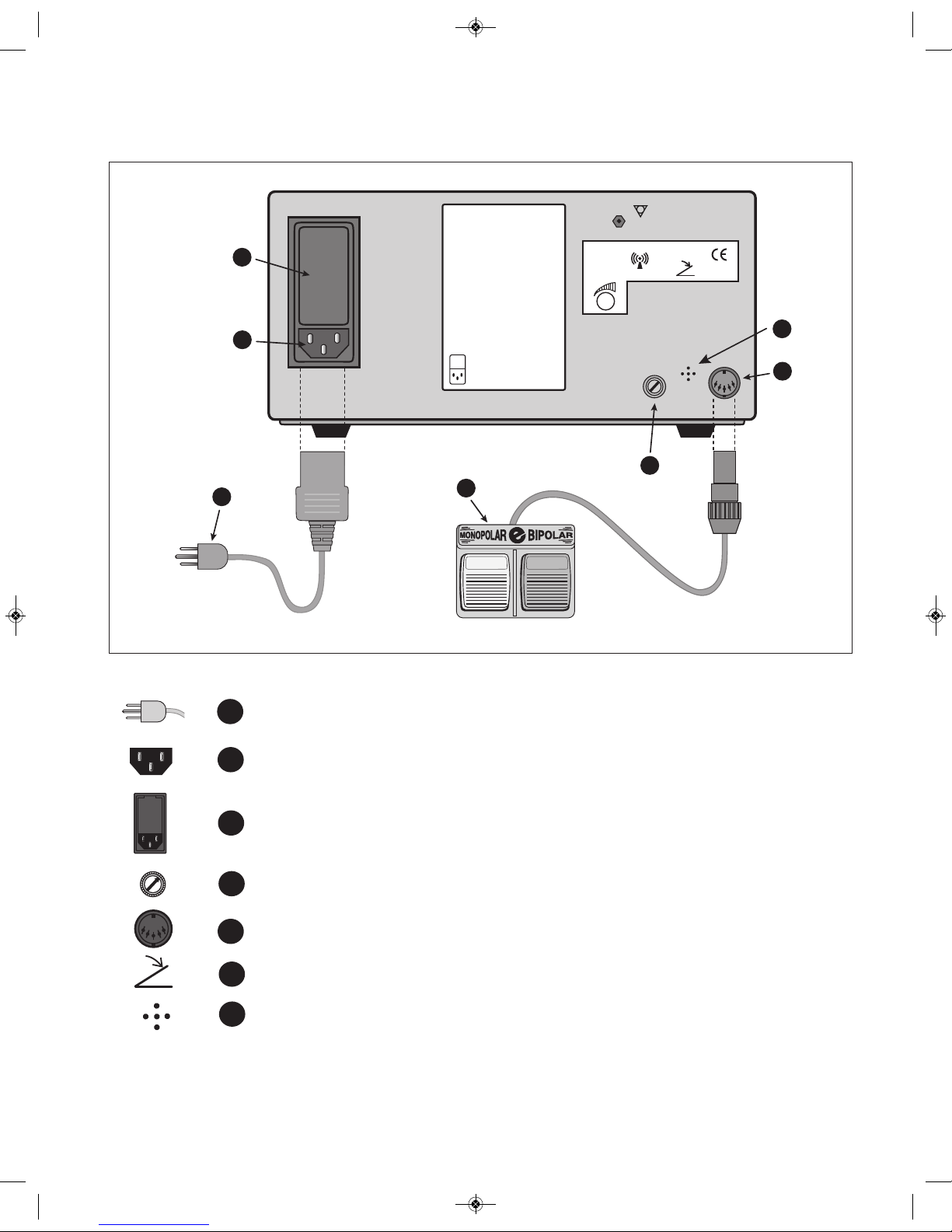
2.0.4 Use only with Ellman accessories including Ellman handpieces, Neutral Pads, cables,
and electrodes.