Denas MS DiaDENS PCM User manual


1
RC ART LLC, Ekaterinburg, Russia
DiaDENS PCM

2
TABLE OF CONTENTS
Part 1. Technical Passport
1. Safety measures..........................................................6
2. Purpose........................................................................10
3. Specications.............................................................11
4. Complete set.............................................................21
5. Device assembly.......................................................22
6. Recommendations
on use of the device....................................................27
7. Using the menu........................................................29
8. Technical maintenance..........................................51
9. Method of replacement of batteries...............52
10. Troubleshooting list.............................................53
11. Manufacturer’s warranty....................................57
Part 2. Usage instruction
1. General provisions...................................................61
2. Indications and contraindications
for use..............................................................................62
3. Conditions for treatment......................................64
4. Intensity (power) of electrostimulation...........65
5.Modalities....................................................................68
6. Treatment with the device...................................70
Appendix 1. Used accessories....................................84
Appendix 2. Scheme of «Express» menu.................89
Warranty certifiсate..................................................107
Acceptance certificate................................................110

3
Thank you for purchasing of the device DiaDENS-PCM.
In order to make the usage of the unit eective and safe, please, carefully read all sections
of this manual.

4

5
PART 1
Technical Passport

6
1. SAFETY REGULATIONS
Information, contained in present operations manual, is important for
your safety and proper use and maintenance of the device.
The device is safe for use, because it utilizes internal power source of low voltage,
which is isolated from work part of the device (article of type B with body of type F).
The device must not be used for treatment of patients with implanted electronic
devices (for example, pacemakers) and for treatment of patients who have
individual electric current intolerance.
Use of the device in direct front projection of heart is prohibited.
7
Don’t treat patient with any high-frequency electric device during stimulation;
simultaneous use of the device and other electric equipment can cause burns
and lead to possible damage of the device.
Work near short-wave and microwave equipment can bring to instability of
output parameters of the device.
You must not connect to the device any other accessories, except remote
electrodes, produced by manufacturing company.
The device contains fragile components. Protect it from shocks.
8
The device is not waterproof. Protect it from ingress of moisture.
All works on maintenance and repair of the device must be executed by qualied
specialists of the manufacturer.
Transport conditions: temperature from -50OC to +50OC, relative air humidity
from 30 to 93%, atmospheric pressure from 70 to 106 kPa (525 to 795 mm Hg).
Storage conditions: temperature from -50OC to +40OC, relative air humidity from
30 to 93%, atmospheric pressure from 70 to 106 kPa (525 to 795 mm Hg.).

9
Operation conditions: temperature from +10OC to +35OC, relative air humidity
from 30 to 93%, atmospheric pressure from 70 to 106 kPa (525 to 795 mm Hg).
Attention! If the device has been stored at the temperature below 10OC, keep it in
normal climate conditions for no less than 2 hours - before use.
Utilization: All packaging materials are not environmentally harmful, they may be
used repeatedly.
Separate collection of electrical and electronic equipment.
The device contains valuable materials, which can be used repeatedly after utilization
with consideration of requirements of environmental protection. They shall be
delivered to specially intended for this purpose places (consult with corresponding
services in your district) for collection and processing.

10
2. PURPOSE
Universal transcutaneous electrostimulator DiaDENS-PCM is intended for application
ofsystem-regulating impacton physiological systems of the body and for the treatment
of functional disordersunder wide range of diseases. Dynamic electroneurostimulation is
executed throughimpulses of electric currenton biologically active points and zones of the
human body.Thedevice provides possibility of selection of individual treatment program, as
well asalready preparedfor use programs. The device has a built-in electrodes and asocket
for connection of remote therapeutic electrodes *.
The device DiaDENS-PCM is intended for use in hospitals and in home conditions in
accordance with the instructions of a physician.
Compliance with standards: This medical device is CE marked according to the
Directive 93/42 /ЕЕС on medical equipment.
You can only connect to the device those remote electrodes, which are produced by the company-manufacturer (see Appendix 1).
11
3. SPECIFICATIONS
3.1. Electric impulse of the device have following output parameters:
3.1.1. Without load:
— amplitude of 1st phase (V) < 40;
— length of 1st phase (uS) - 22 ± 25%;
— amplitude of 2nd phase (V) - 72 ± 25%.
Comments: parameters of impulse do not change under adjustment of power and
frequency.
3.1.2. With load = 20 kOhm
Power (in nominal units) Minimum Maximum
1 99
Amplitude of 1st phase, V ≤ 40 ≤ 40
Length of 1st phase, uS 6 ± 25% 530 ± 25%
Amplitude of 2nd phase, V 9 ± 25% 300 ± 25%
12
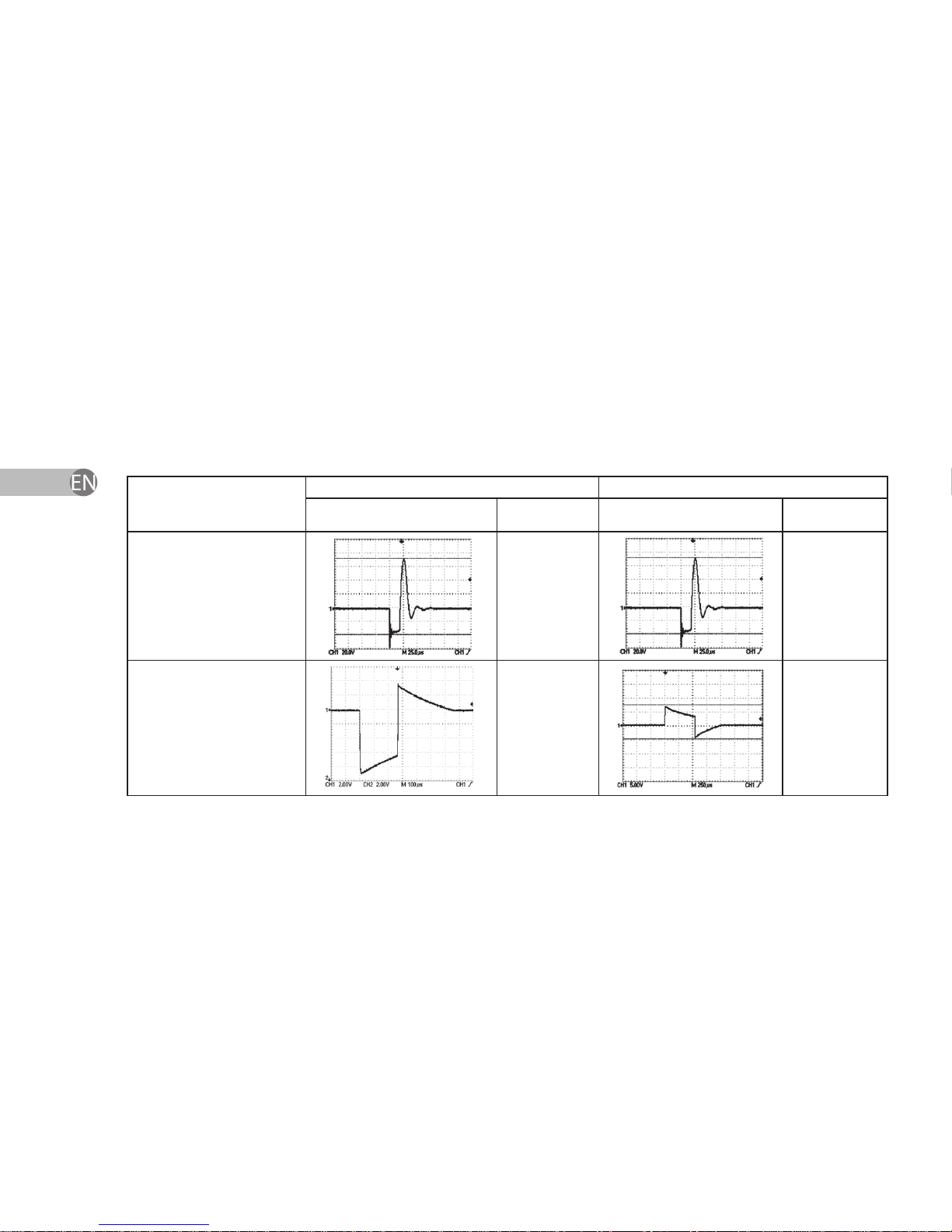
3.1.3. Dependence of impulse form from load resistance at average and maximum
power levels.
Load resistance Power level 50 units Power level 99 units
Form of current Vp-p Form of current Vp-p
Without
load
≈110 V ±
20 %
≈110 V ±
20 %
200 Ohm ≈12 V ±
20 %
≈12 V ±
20 %

13
500 Ohm
≈29 V ±
20%
Ie =16
mA
Epulse≈110
uJ
≈30 V ±
20 %
Ie = 17 m
Epulse≈205
uJ
1 kOhm ≈52 V ±
20 %
≈60 V ±
20 %
2 kOhm ≈90 V ±
20 %
≈100 V ±
20 %

14
10 kOhm ≈282 V ±
20 %
≈300 V ±
20 %
20 kOhm ≈310 V ±
20 %
≈330 V ±
20 %
3.2. The device provides possibility of setting of following frequencies of impulses, Hz:
3.2.1. Range 1:
— 10±1, including modes «Experess», MED (prophylaxis) and 14 «Screening»;
15
— 20±1; 60±2; 77±2; 77±2 and 10±1, 77±2 and 10±1 modulated by frequency
2±0,1 (mode «77 10»);
— 77±2 with modulation by amplitude (mode «77AM»); 140±3; 200±3.
3.2.2. Range 2: from 1,0 to 9,9 with increment 0,1±0,05.
3.3. Maximum current consumption (under supply voltage of 3 V - no more than 300 mA.
3.4. Electrical power supply: batteries of type LR6/AA, 2 pcs., with voltage 1,5±0,45 V. It is
allowed to use corresponding accumulator batteries with nominal current of 1,2 V*.
3.5. Mass of the device is no more than 0,35 kg.
3.6. Dimensions of the device are no more than 165
65
65 mm.
Operations procedure (types of battery rechargers, methods of recharge) are described in instructions manual
for accumulator batteries. Running time of the device under use of accumulators depends on characteristics of
accumulators.

16
3.7. The device automatically switches o not later than in 10 min after the last touch of any
of its buttons (except button ) or after the last contact of the electrodes with patient’skin
surface.
3.8. Electromagnetic emission
Test Compliance with IEC 60601-1-2 Terms of use
RD radiation
CISPR 11 Class B Electrosimulator DiaDENS-PCM is suitable for
use in all houses including residential use
3.9. Resistance to RF-radiation
Test IEC 60601-1-2 test conditions Acceptable level
IEC 61000-4-6 3 Vrms from 150 kHz to 80 mHZ 3 Vrms
IEC 61000-4-3 3 V/m from 80 MHz to 2,5 GHz 3 V/m
17
3.10. Resistance to electromagnetic elds
Test Test level Level of
compliance Terms of use
Electrostatic
discharge
(ESD) IEC
61000-4-2
±6 kV
contact ±8
kV impact
±4 kV
contact ±8
kV impact
Floor should be made from wood, concrete, or
ceramic tiles. If oor is covered with synthetic
material, the relative humidity should be at
least 40%.
Magnetic
elds
IEC 610004-8
3 A/m 3 A/m
Parameters of magnetic elds should be
at levels, typical for commercial or hospital
buildings

18
3.11. Recommendations on determination of required distance between electrostimulator
DiaDENS-PCM and radio equipment
Rated maximum output power of
Transmitter P (W)
Radiated frequency and formula for
determination of distance d (m)
150 khz - 80 MHz
d = 1,2VP
150 khz - 800 MHz
d = 1,2VP
800 MHz - 2,5 HHZ
d = 2,3VP
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
3.12. Terms of use
3.12.1. Connection of remote electrodes, not allowed by manufacturer (including
change of length of cable, type of cable, construction of electrode) can lead to increase
of electromagnetic radiation level and/or decrease of device resistance to external
impacts.

19
3.12.2. The device DiaDENS-PCM uses electromagnetic energy only for internal
functions, and due to this fact, radiation of the device is minimal and shall not impact
nearest electronic equipment. The device DiaDENS-PCM shall not be used alongside
other equipment. If such action is deemed necessary, then DiaDENS-PCM and other
equipment shall be checked for correctness of operation under joint use.
3.12.3. The device DiaDENS-PCM is intended for work in specic conditions of
electromagnetic environment, and the customer (user) shall check if these conditions
comply with required values.
Electrostatic discharge (ESD). Floor should be made from wood, concrete, or ceramic
tiles. If oor is covered with synthetic material. Then relative air humidity shall be no
less than 40%.
RF-radiation. Portable and mobile devices shall be used near any part of the device
DiaDENS-PCM at the distance of no less than d = 2,3 V P (800 MHz + 2,5 HHz), where P –
is maximum output power according to manufacturer’s information.
Table of contents
Other Denas MS Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual

agiliti
agiliti NP Trio user manual

Rocket Medical
Rocket Medical Rocket Digital Oocyte Aspiration Pump Service manual

Monteris Medical
Monteris Medical Neuroblate Instructions for use

Otto Block
Otto Block 8E33 7 manual

ADVANCED HOME CARE
ADVANCED HOME CARE Helios Guide

NeuLog
NeuLog NUL-208 Guide

Healthtree Medical
Healthtree Medical JKS50A Operator's manual






