Diaglobal Vario Photometer II User manual

1
2021-01
Vario Photometer II
DP 310
Operating Manual
Version 5.13 / 5.13 SI
Edition 2021-01

2
2021-01
Dear customer,
We are pleased that you have chosen the Vario Photometer II from Diaglobal GmbH and
thank you for the confidence you have placed in us.
The Vario Photometer II belongs to a new generation of small mobile devices developed
by Diaglobal GmbH and specially designed for on-site analysis.
With the software version V5.3 and higher, an automatic test of the device function has
also been integrated. Therefore, the Vario Photometer II complies with the requirements
of the guidelines of the German Medical Association.
With the Vario Photometer II, 13 clinical-chemical parameters can be determined.
The device can be supplied with SI units of measurement on request (see chapter 9,
Technical Data, table Measuring Ranges).
The kits and accessories required for the test are also available from Diaglobal GmbH.
All the best for your work with the new Vario Photometer II!
Yours
Diaglobal GmbH

3
2021-01
Table of contents
Page
1. General information regarding the Photometer 4
2. Installation 5
3. Description of the device 5
3.1 Power supply 6
3.1.1 Mains power operation 6
3.1.2 Mains-independent operation 6
3.2 Measuring system 6
4. Service 7
4.1 Adjustment and Calibration 7
4.2 Maintenance 7
4.3 Cleaning Instructions 7
4.4 Malfunctions 7
4.5 Disposal 7
5. Required reagents and laboratory accessories 8
5.1 Expiration date of consumables 8
5.2 Reagents / parameter list 8
5.3 Control materials 9
5.4 Laboratory aids and accessories 9
6. Quality control according to the Guideline of the German Medical
Association 10
7. Measuring procedure 11
7.1 End-point measurement 11
7.2 End-point measurement, taking the sample’s blank value and
pre-programmed measuring time into account 11
7.3 Multipoint measurement, taking the sample’s blank value and
recognition of the end point into account 12
7.4 Multipoint measurement, taking the sample’s blank value and
calculation of the end point into account 12
7.5 2-point kinetics (fixed time) reaction with fixed incubation time and
fixed measuring time 12
7.5.1 Creatine Kinase from blood <CK 321> 12
7.5.2 Creatine Kinase from serum/plasma <CK 121> 13
8. Measurement 14
8.1 Switching the device on 14
8.2 Self-test when switching on 14
8.3 Test selection 14
8.4 Switching the device off 14
8.5 Integrated operational device checks 14
8.6 Notes on taking samples and carrying out measurements 15
9. Technical data 17
10. General guidelines and notes 18
11. Appendix: “Step-by-step measurement” 18ff.

4
2021-01
1. General information on the Photometer
Device name: Vario Photometer II
Model: DP 310
Features: In-vitro diagnostics, measuring device for the
determination of selected clinical-chemical
parameters in blood, serum/plasma and
cerebrospinal fluid
The Vario Photometer fulfils the basic requirements of Appendix I of Directive
98/79/EC regarding in-vitro diagnostics.
The conformity of the device with Directive 98/79/EC is confirmed by the use of
the CE marking.
Manufacturer: Diaglobal GmbH
Innovationspark Wuhlheide
Köpenicker Str. 325 / Haus 41
12555 Berlin
Tel: +49 (0) 30 6576 2597
Fax: +49 (0) 30 6576 2517
E-Mail: info@diaglobal.de
http://www.diaglobal.de

5
2021-01
2. Installation
For trouble-free operation of the device, the following environmental conditions
must be met:
• Ambient temperature: 0 °C ... 40 °C
• No direct exposure to sunlight or similar sources of radiant heat
• Free from excessive dust
• Free from vibrations
• Free from interference by electromagnetic waves
• Operation on a horizontal surface
Please observe the following instructions for use:
Insert a rechargeable battery or normal battery if the device is to be operated
independently of a power supply or connect the photometer to a power supply
unit.
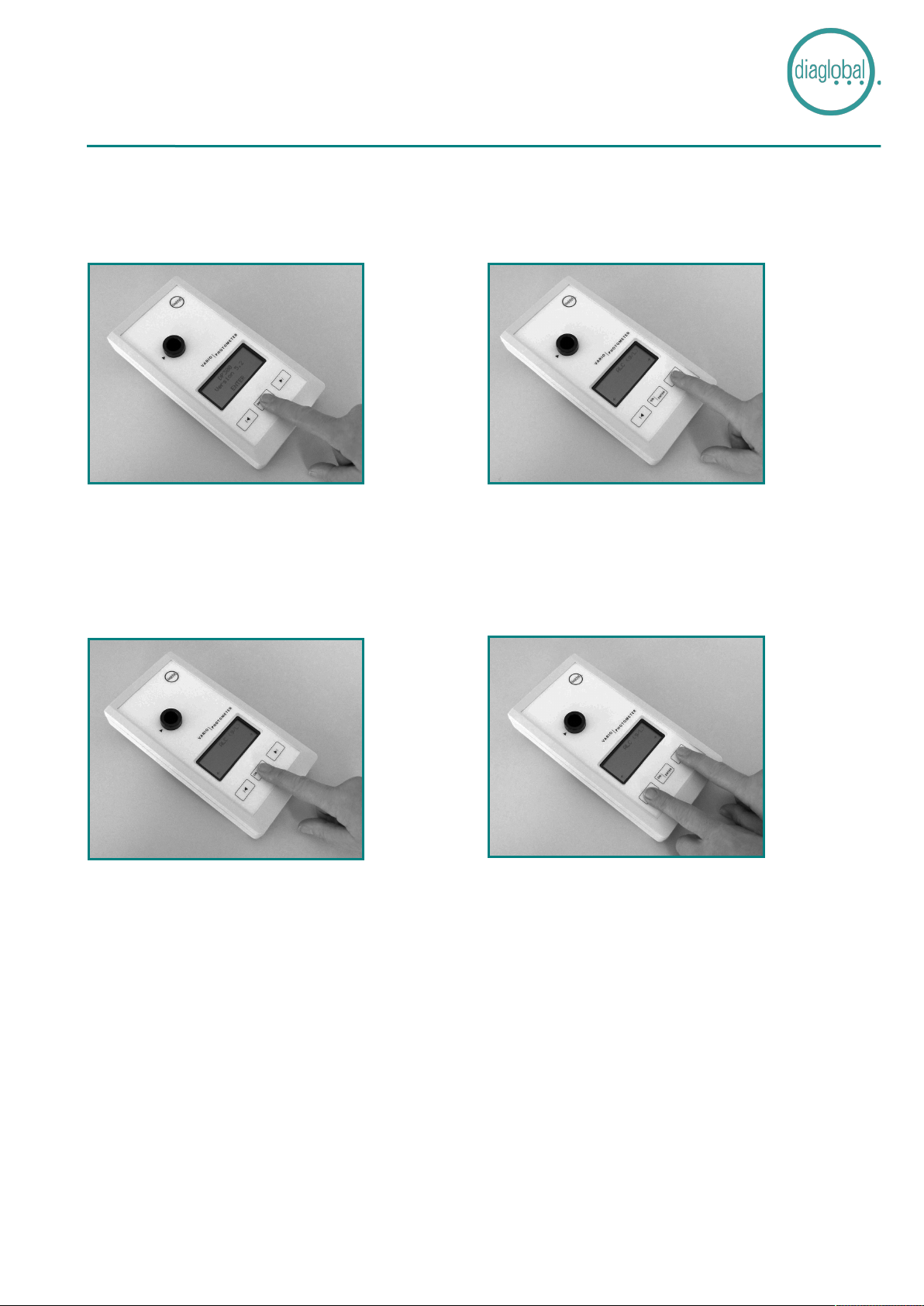
Press the <ON/ENTER> key (Fig. 1) to activate the internal device check which
is automatically carried out by the device.
The device is then immediately ready for measurement.
3. Description of the device
Cuvette shaft
Display
Function keys
Fig. 1

6
2021-01
3.1 Power supply
The Vario Photometer II can be operated as desired using a power supply, a (9V
block) battery or (model 6F22 or PP3) rechargeable battery.
3.1.1 Mains power operation
The Photometer is supplied with a power supply unit for operation on a mains
voltage in the range of 100 V ... 240 V AC. The mains plug is marked with a
Diaglobal logo (sticker).
The connector plug of the power supply unit is connected to the power supply
socket on the back of the device.
3.1.2 Network-independent operation
To insert the rechargeable battery or the normal battery:
Unscrew the knurled screws on the bottom of the unit and remove the battery
compartment cover. Connect the battery to the push-button contact and insert it
into the device. Replace the battery compartment cover and screw in the knurled
screws.
Please note:
The Vario Photometer plus can be operated using a power supply without the need
to remove the rechargeable battery or the normal battery.
The rechargeable battery cannot be charged while it is installed. A separate
battery charger is required for this purpose.
3.2 Measuring system
The optical section is shown in Fig. 2.
Fig. 2
The light emitted by an LED is first selected into its wavelength ranges (365 nm
and 520 nm) by an interference filter IF (HBW ~ 5 nm) and then bundled and
directed onto the cuvette in the shaft. After passing through the cuvette, a
broadband photosensor converts the light falling on its sensor surface into a
current, proportional to the intensity.

7
2021-01
4. Service
4.1 Adjustment and Calibration
The instrument is adjusted and calibrated at the factory on delivery, adjustment
by the customer is not necessary.
Adjustment is carried out via the interface socket on the rear panel. It can only be
carried out at the factory, adjustments by the customer are not possible.
Information on calibrating the device can be found in chapter 6. Quality control
according to the Guideline of the German Medical Association.
4.2 Maintenance
The device is maintenance-free. Maintenance after the warranty period is
recommended, but not mandatory.
Due to the integrated test of the unit functions (chapter 8.5) and regular tests
with control material, maintenance is only recommended if one of these two test
functions indicates an error message.
4.3 Cleaning Instructions
Commercially available decontaminating solutions commonly used in clinical
chemistry laboratories, such as Mikrozid® AF Liquid, Bacillol® plus, 3 %
Kohrsolin® or similar, are recommended for cleaning the device and the surface.
Before cleaning the unit with a soft cloth and the decontaminating solution, it
must be switched off and the electrical power supply must be disconnected.
Make sure that no liquids get into the device. There is no protection against
penetrating liquids (Code IP X0).
The cuvette shaft must not be cleaned by the user of the device, as this may
damage the device. If cleaning is necessary, especially because of leaking liquids
or broken glass, please contact Diaglobal GmbH.
4.4 Malfunctions
If any malfunctions or problems occur, simply call us. Most questions can be
answered on the phone. Non-functional units should be sent to our Berlin address.
We will provide a loan device for the duration of the repair.
4.5 Disposal
Diaglobal GmbH will take back and dispose of units that are no longer needed or
cannot be repaired, free of charge.

8
2021-01
5. Required reagents and laboratory accessories
5.1 Expiration date of consumables
It is important to ensure that all consumables may only be used within the
expiration date.
5.2 Reagents / parameter list
The following tests can be measured with the Vario Photometer II:
Parameter
Sample material
Tests/pack
Art. no.
Blood Serum Plasma
CK from blood1) 2) 3) + + + 20 CK 321
CK from serum2) - + + 20 CK 121
GOT/ASAT2) - + + 40 GOT 442
GPT/ALAT2) - + + 40 GPT 442
Lactate + - + 40 LAC 142
Lactate-rapid + - + 40 LAC 342
Urea1) 3) + + + 20 HST 321
Glucose + + + 40 GLU 142
Triglycerides + + + 40 TRI 142
HDL-Cholesterol1) 3) + + + 20 HDL 321
Cholesterol + + + 40 CHO 142
Creatinine2) - + + 20 KRE 121
Haemoglobin (SLS-method) + - - 40 HB 342
Erythrocytes + - - 40 ERY 142
Haematocrit + - - 40 HCT 142
1) Mini centrifuge required (Art. no. DZ 002)
2) Dry Block Thermostat required (Art. no. DZ 003)
3) From blood, with subsequent sample preparation (centrifugation with mini centrifuge)

9
2021-01
5.3 Control materials
Art. no.
Description
Contents
HEM QS Haemoglobin control
Haemolysate for correctness and precision control of haemoglobin
determination in blood in the normal range
5 x 1 mL
ERY QS Erythrocytes- and Haematocrit control
Control blood for accuracy and precision control of haematocrit and
erythrocytes determination in blood in the normal range
5 x 1 mL
GLU QS Glucose control 100 mg/dL 3 x 4 mL
LAC QS Lactate control set 2 mmol/L ; 4 mmol/L ; 10 mmol/L 3 x 4 mL
5.4 Laboratory aids and accessories
Art. no.
Description
Contents
LH 001 Blood lancets 500
LH 004 Capillaries 10 µL, with ring mark 250
LH 006 Cuvette rack 1
LH 007 Micropipettor (pipetting aid) 1
LH 009 Cellulose swabs 500
LH 010 Cellulose swab box 1
LH 011 Alcohol pads, non-sterile 100
LH 012 Powder-free nitrile gloves size M 200
LH 020 Capillaries 20 µL, heparinised, end-to-end 100
LH 024 Capillaries 20 µL, with ring mark 250
LH 035 Safety lancets extra, orange 1.8 mm 200
LH 050 Reaction tubes to separate the plasma 500
LH 055 Pipette tips 50-1000 µL blue, for pipette LH 500 500
LH 056 Capillaries 50 µL, end-to-end 100
LH 060 Capillaries 60 µL, heparinised, end-to-end 5 x 20
LH 500 Pipette fix 500 µL 1
DZ 002 Mini centrifuge 1
DZ 003 Dry Block Thermostat 1
All reagent kits, control materials and other materials are supplied by Diaglobal
GmbH and can be stored and transported together with the Vario Photometer II in a
practical case.

10
2021-01
6. Quality control according to the Guideline of the
German Medical Association1)
The Vario Photometer II has been specially developed for near-patient immediate
diagnostics with unit-use reagents (German Medical Association, part B, chapter
2.1.5). According to the guideline of the German Medical Association, there is
therefore no obligation to participate in surveys (German Medical Association,
part B, chapter 2.2, paragraph (3) a). The user only has to carry out internal
quality checks.
Internal quality assurance is carried out in the form of a weekly accuracy check
(calibration) with subsequent documentation of the measured value. The
corresponding protocol forms are available from Diaglobal free of charge.
We recommend using the Diaglobal control solutions LAC QS and GLU QS to check
the accuracy of lactate and glucose determinations.
We recommend using the blood control HEM QS and ERY QS with target values in
the normal concentration area for checking the accuracy of determinations of
haemoglobin, haematocrit and erythrocyte counts.
For all other parameters we recommend using the universal control sera from the
company, Roche, www.roche.de:
PreciControl ClinChem Multi 1 Order-No.: 05 947 626 190 (4 x 5 mL) for normal range
PreciControl ClinChem Multi 2 Order-No.: 05 947 774 190 (4 x 5 mL) for pathological range
In agreement with the requirements of the German Medical Association, a test of
the device function (see operating instructions, chapter 8.5) is integrated in the
Vario Photometer II, therefor a daily test by means of a standard manual test
(German Medical Association, part B, chapter 2.1.5, paragraph (2) is not
necessary.
The Vario Photometer II is suitable for the speedy detection of gestational
diabetes and fulfils the requirements of the Maternity Guidelines2) and the S3-
Directive3). Glucose can be measured from whole blood as well as from venous
plasma. The displayed measured value is - according to the requirements - always
related to venous plasma.
1) Guideline of the German Medical Association for the quality assurance of laboratory medical examinations
Deutsches Ärzteblatt | Jg. 116 | Heft 51-52 | 23. Dezember 2019
2) BAnz. Nr. 36, S914
3) AWMF-Register Nr. 057/008

11
2021-01
7. Measuring process
7.1 Endpoint measurement
The absorbance is measured after reaching the endpoint.
It is measured against the reagent’s blank count.
Parameters: Haemoglobin SLS (HB SLS), Erythrocytes (ERY), Haematocrit (HCT),
HDL Cholesterol (HDL/CHO)
Calculation: Concentration = Absorbance x Factor
The erythrocyte and haematocrit counts are determined using stored reference
curves.
7.2 Endpoint measurement with consideration of the sample blank value and
pre-programmed measuring time
After measuring the sample blank value, the colour reaction in the cuvette is
started and the endpoint absorbance is measured after a specified time has
elapsed.
Parameter: Creatinine (CRE)
Calculation: Concentration = Absorbance x Factor
Measuring time: 2 minutes
The samples are measured one after the other:
Sample 01: Measurement 1 (sample blank value)
Sample 01: Measurement 2 (result)
Sample 02: Measurement 1 (sample blank value)
Sample 02: Measurement 2 (result)
etc.
Parameter: UREA
Calculation: Concentration = Absorbance x Factor
Measurement time: 10 minutes
Blank values of the samples are measured one after the other:
Sample 01: Measurement 1 (sample blank value)
Sample 02: Measurement 1 (sample blank value)
Sample 03: Measurement 1 (sample blank value)
etc.
Results are measured one after the other:
Sample 01: Measurement 2 (result)
Sample 02: Measurement 2 (result)
Sample 03: Measurement 2 (result)
etc.

12
2021-01
7.3 Multi-point measurement with consideration of the sample blank value
and recognition of the endpoint
After measuring the sample blank value (=measurement 1) the colour reaction in
the cuvette is started. The reaction process is monitored by the device
(=measurement 2). The measuring process is terminated as soon as the endpoint
is reached.
The time needed to reach the endpoint depends on the temperature. It is normally
2 - 6 minutes for the lactate test. If temperatures are close to freezing point,
measuring times can take up to 20 minutes, depending on the parameters.
You can choose between single and series measurements up to a maximum of 20
samples.
For single measurements, the samples are processed one after the other.
For series measurements, all A1 values are measured first.
Parameters: Lactate (LAC), Cholesterol (CHO), Triglycerides (TRI)
Calculation: concentration in plasma = Absorbance Difference x Factor
7.4 Multi-point measurement with consideration of the sample blank value
and calculation of the endpoint
After measuring the sample blank value (=measurement 1) the colour reaction in
the cuvette is started. The course of the reaction is monitored by the instrument.
The endpoint is calculated using several absorbance values recorded at different
times.
Parameters: Glucose (GLU), Lactate-rapid (LAC-rapid)
Measuring times: Glucose 2 minutes
Lactate-rapid 1 minute
7.5 2-point kinetic (fixed time interval) reaction with fixed incubation time
and fixed measuring time, T = 37°C
7.5.1 Creatine Kinase from blood <CK 321>
The reaction is started in the cuvette with the starter cap. The measurement
starts simultaneously on the photometer by pressing the <ON/ENTER> key.
After incubation of 5 minutes, the first absorbance (A1) is measured. After
another 10 minutes, the second absorbance (A2) is measured. For series
measurement, the photometer specifies a time interval (15 seconds). For series
lengths of N<6, the <ON/ENTER> key must be used to switch to the A2
measurement. For N=6, the device performs the switchover automatically. The
measured enzyme activities of the individual samples can be accessed with the
arrow keys.
Calculation: The Creatine kinase concentration is calculated from the difference of
the absorbances (ΔE) using a calibration factor. Because the determination is
made from blood, the haematocrit value must be considered.
Enzymatic activity (U/L) = Factor x (ΔA)/(1-0,01*HCT)

13
2021-01
Individual haematocrit values can be considered, they will be requested before
calculation. The default setting is an HCT value of 40%.
Please note: If the sample material is serum/plasma, the HCT value must always
be set to 0%.
7.5.2 Creatine Kinase from serum/plasma <CK 121>
The reaction in the cuvette is started by adding the sample. The time
measurement starts simultaneously on the photometer by pressing the
<ON/ENTER> key. After incubation of 5 minutes, the first absorbance (A1) is
measured. After another 10 minutes, the second absorbance (A2) is measured.
For series measurement, the photometer specifies a time interval (15 seconds).
For series lengths of N<6, the <ON/ENTER> key must be used to switch to the
A2 measurement. For N=6, the device performs the switchover automatically. The
measured enzyme activities of the individual samples can be accessed with the
arrow keys.
Calculation: The creatine kinase concentration is calculated from the difference of
the absorbances (ΔE) using a calibration factor.
Enzyme activity (U/L) = factor x ΔE

14
2021-01
8. Measurement
8.1 Switching the device on
Press the <ON/ENTER> key
8.2 Self-test when switching on
When the device is switched on, a self-test of the digital and analogue circuitry is
conducted. The operational device check proceeds automatically after it is
switched on. It takes approx. 5 seconds, after which the unit is ready for
measuring.
Note:
If it becomes obvious during the test that one of the device functions does not
correspond to the required settings, <SERVICE> will appear in the display.
In this case, switch the device off.
Please call Diaglobal GmbH service (Tel. +49 (0) 30 6576 2597) or contact your
specialist retailer.
8.3 Test selection
Press the <ON/ENTER> key.
The desired test is selected from the menu with the right or left arrow key:
CK 321 - CK 121 - GOT - GPT - LAC - LAC-rapid - HST - GLU - TRI - HDL/CHO -
CHO - KRE - HB-SLS - ERY - HCT - ABS365 - ABS520
Pressing the right arrow key activates the next test while pressing the left arrow
key returns to the previous test. The selected test is shown in the upper right
corner of the display.
Confirm test selection with the <ON/ENTER> key.
8.4 Switching the device off
To switch the device off, press both arrow keys simultaneously.
8.5 Integrated operational device checks
Self-test when switching on
Testing of the digital and analogue circuits of the device is automatically
performed by the device when it is switched on.
Please see chapter 8, point 8.2.
Differential measurements
All measurements are based on differential measurements. I. e. after selecting the
desired test, the device requests a zero measurement with a blank value cuvette.
This creates a reference base to the measured value so that minor deviations can be
compensated.

15
2021-01
Measurement range controls
The measurement ranges of all measurement results shown in the display are
verified by an integrated measurement range control. If the measurement range is
exceeded, an error is displayed.
The measurement ranges that are separately defined for each parameter
are documented on the respective package inserts as well as in this
operating manual, chapter 9, Technical Data.
Plausibility controls
For multi-point measurements, the absorbance measured first forms the
reference basis. The programme verifies the plausibility of the individual
measured values. If specific requirements (e.g. E2 > E1 for ascending reactions)
are not met, an error message is displayed.
8.6 Notes on taking samples and carrying out measurements
Errors in taking samples will always lead to incorrect measurement results.
This chapter addresses the most common errors that can occur during taking
samples and measuring samples.
1. Before measuring, cuvettes stored in a refrigerator must be brought to room
temperature. If the cuvettes are too cold, they will become misty with water
on the outer wall due to the humidity, which will lead to incorrect
measurement results.
2. Never touch the lower part of the cuvette (where the liquid is) with bare
hands. If this should happen accidentally, clean the vials with a fluff-free cloth
before use. Cleaning with a fluff-free cloth is recommended in any case. Even
if the package is still new and unopened. Fingerprints on the cuvette lead to
incorrect measurement results.
3. If blood is taken from the fingertip or earlobe, note that the first drop that
forms spontaneously must be wiped away with a cellulose swab. It contains a
high proportion of tissue fluid, which will corrupt the measurement result.
4. The second drop that forms is for blood sampling. To support blood collection,
it may be pressed carefully (!). The emphasis on carefully, otherwise too much
tissue fluid will get into the blood sample again.
5. Make sure that the blood drop that forms is large enough to fill the capillary
with the required sample volume in one go. Repeated filling of the capillary
leads to air bubbles that cannot be removed from the capillary. If air bubbles
form, discard the capillary and start sampling again.
6. The capillary must be filled exactly up to the black ring mark.
Please note: A deviation of only 1 mm from the ring mark is sufficient to
obtain a completely incorrect measurement result!
If the sample is above the black ring mark, this will lead to incorrect positive
measurement results. A cellulose swab can be used to carefully soak up too
much blood.

16
2021-01
If the sample is below the black ring mark, this will lead to incorrect negative
measurement results. In this case, correction is hardly possible due to the air
bubble that will form when trying to collect more blood.
7. Before the capillary is placed in the cuvette, the lower area must be carefully
wiped on the outside with a cellulose swab to remove sample particles
attached to the capillary. Otherwise, this would lead to incorrect positive
measurement results.
8. With the help of the micropipetter, the sample is completely transferred into
the cuvette. The complete transfer of the sample is done by ejecting it several
times with the help of the push button on the micropipetter.
Please note: The micropipetter is only used when the capillary is filled with the
sample. It is not needed for filling the capillary. The capillary is filled by the
capillary action alone.
9. During series measurements, make sure that the order of the samples is not
reversed. Otherwise, the device cannot assign the samples correctly, which
leads to unreliable measurement results.
10. When changing the cap with the starter cap, make sure that the substance in
the starter cap has completely dissolved. Failure to do so will result in a non-
linear kinetic reaction process, which will lead to an error message in the
display or unreliable measurement results.

17
2021-01
9. Technical data
Storage temperature: -20 °C ... 70 °C
Operating temperature: 0 °C ... 40 °C
Dimensions: 200 x 100 x 50 mm
Weight: 450 g
Measuring principle: Absorption measurement with single beam
photometer (Fig. 2), chopped operation
Projector: LED
Spectral apparatus: Interference filter
Measuring wavelengths: 365 nm and 520 nm
Spectral half-width value: ~ 5 nm
External light influence: Negligible
Interface: V24 (9600, 8, n, 2)
Power supply: 6 V ... 12 V DC
Current consumption: max. 250 mA
Warm-up time: 0 min
Interference suppression: According to DIN VDE 0871 and DIN VDE 0875
Inaccuracy: < 0.5 % at A = 1.000
Relative photometric
short-time standard deviation: < 0.1 %
Measuring ranges: DP 310 DP 310 SI
CK in blood / CK 321 0.0 - 2500 U/L 0.0 - 2500 U/L
CK in serum / CK 121 0.0 - 2000 U/L 0.0 - 2000 U/L
GOT (ASAT) 10 - 500 U/L 10 - 500 U/L
GPT (ALAT) 10 - 500 U/L 10 - 500 U/L
Lactate 0.2 - 30 mmol/L 0.2 - 30 mmol/L
Lactate-rapid 0.2 - 20 mmol/L 0.2 - 20 mmol/L
Urea 5 - 200 mg/dL 0.8 - 35 mmol/L
Glucose 20 - 630 mg/dL 1.1 - 35 mmol/L
Triglycerides 20 - 2000 mg/dL 0.2 - 23 mmol/L
HDL-Cholesterol 10 - 200 mg/dL 0.2 - 5 mmol/L
Cholesterol 20 - 1300 mg/dL 0.5 - 35 mmol/L
Creatinine 0.0 - 5 mg/dL 0.0 - 440 µmol/L
Haemoglobin (SLS-method) 0.0 - 50 g/dL 0.0 - 31 mmol/L
Erythrocytes 1.0 - 10 Mio/µL 1.0 - 10 Mio/µL
Haematocrit 5 - 90 % 5 - 90 %
ABS 365 nm A = 2.500 A = 2.500
ABS 520 nm A = 2.500 A = 2.500

18
2021-01
10. General Guidelines and Notes
EC Directives
1. Directive 98/79/EC on in-vitro diagnostic devices
EN / ISO standards
2. EN ISO 9001:1994, Quality Management Systems, Model for quality
assurance in design, development, production, installation and customer
service
3. EN ISO 13485, Medical devices, Requirements for regulatory purposes
(application of EN ISO 9001)
4. EN ISO 14971, Medical devices - Application of risk management to
medical devices
5. EN 61010 -1, Safety requirements for electrical equipment for
measurement, control and laboratory use - Part 1: General requirements
6. EN 61010 -2-101, Safety requirements for electrical equipment for
measurement, control and laboratory use - Part 2-101: Particular
requirements for in-vitro diagnostic (IVD) medical equipment
7. EN 61326 -1, Electrical equipment for measurement, control and
laboratory use - EMC requirements - Part 1: General requirements
8. EN 61326 -2-6, Electrical equipment for measurement, control and
laboratory use - EMC requirements - Part 2-6: Particular requirements –
In-vitro diagnostic (IVD) medical equipment
9. EN 592, Instructions for use for in-vitro diagnostic instruments for
professional use
National directives and recommendations (Germany)
10. Guidelines for Quality Assurance of Laboratory Examinations of the
German Medical Association of 23.12.2019
Note on electromagnetic compatibility
a) The photometer meets the requirements for electromagnetic radiation
and interference immunity as described in the IEC 61326 series of
standards.
b) Do not use this device near sources of intense electromagnetic radiation
because they may interfere with correct functioning. A distance of at
least 1 m should be maintained between an operational (switched on)
mobile phone and the photometer during measurement.
Note on the unit's internal quality control
The functionality of the device is checked when it is switched on. In addition,
electronically controlled checks are carried out for individual tests during the
measurement, which leads to an error message if specified requirements are
not met.
11. Appendix: “Step-by-step measurement”
Please refer to the illustrations in the "Step by step" instruction manual.
Step by step instructions
Device manual
Diaglobal GmbH · Köpenicker Straße 325 · 12555 Berlin · +49 (0)30 6576 2597 · info@diaglobal.de · www.diaglobal.de 2021-01
1. Switch on:
Press ON/ENTER key
Wait for device check and confirm with
ON/ENTER
2. Select test:
Press arrow key until required test
appears
3. Confirm required test:
Press ON/ENTER
4. Switch off:
Press both arrow keys at the same time
Note:
If SERVICE appears in the display after
the device check, the device has a defect.
In this case, please contact our customer
service at +49 (0) 30 6576 2597.

Step by step instructions
CK 321
Number of samples per series: Up to 6 samples at the same time
Additionally required: Dry block thermostat (30 minutes preheated), Mini centrifuge, Haematocrit HCT 142
Diaglobal GmbH · Köpenicker Straße 325 · 12555 Berlin · +49 (0)30 6576 2597 · info@diaglobal.de · www.diaglobal.de 2021-01
1. Transfer 60 µL of the sample with
an end-to-end capillary into each
reaction tube "R" and mix well
Note: The hematocrit value should be
known or must have been measured
previously with HCT 142
2. Insert reaction tube „R“ with
capillary into mini centrifuge
Centrifugate for 1 minute
Note: Ensure an even loading inside
the mini centrifuge
4. Screw starter cap on and mix
extremely well
Then insert the cuvette
immediately into the dry block
thermostat
6. Start measurement with ON/ENTER
Time (5 minutes) counts backwards. All
cuvettes remain in the dry block
thermostat during this time
Double signal tone: The M1
measurement starts in 30 seconds!
5. Switch photometer on with
ON/ENTER key
Wait for device check, confirm
with ON/ENTER
Select CK 321, confirm with
ON/ENTER
ON
ENTER
ON
ENTER
CK 321
CK Measurement
START
7. Follow the display, insert sample 1
into the photometer, „Measuring“ is
displayed, wait for 10 seconds
Then remove the cuvette and incubate
it again
Proceed in the same way with all other
cuvettes in the correct order
Then press ON/ENTER
8. Time (10 minutes) counts backwards.
All cuvettes remain in the dry block
thermostat during this time
Double signal tone: The M2
measurement starts in 30 seconds!
For the M2 measurement follow the
display in the same way as discribed in
Fig. 7
ON
ENTER
M2 CK 321
Sample 09:30
incubate
M1 CK 321
Samples 04:30
incubate
ON
ENTER
M1 CK 321
Sample 01 00:03
insert
3. Pipette 500 µL supernatant
from the reaction tube "R" into
the cuvette
ON
ENTER
CK 321
Sample 01
HCT: 40%
- ENTER +
9. After inserting the last cuvette, the
request to enter the HCT values for each
sample is displayed
Enter the known or previously measured
HCT values with the right or left arrow key
and confirm with ON/ENTER
After entering the last HCT value, read all
measured values one after the other by
pressing the right arrow key
This manual suits for next models
1
Table of contents
Other Diaglobal Diagnostic Equipment manuals
Popular Diagnostic Equipment manuals by other brands
Filtrine
Filtrine QCP Installation, operation and maintenance instructions

Autel
Autel MaxiCheck MX808 manual
Siemens
Siemens Intelli-Arc instruction sheet

MSW Motor Technics
MSW Motor Technics MSW-CA-115 user manual

TecMate
TecMate VacuumMate Allweather Service manual
TRACER PRODUCTS
TRACER PRODUCTS Marksman II Series Operator's manual