Diamedica DPA 02 Manual

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 1of 25
DPA 02TM
Diamedica Portable Anaesthesia System
INSTRUCTIONS FOR USE MANUAL
ENG
Diamedica (UK) Ltd
Grange Hill Industrial Estate
Bratton Fleming, Barnstaple,
Devon, EX31 4UH, United Kingdom
Tel: +44 (0)1598 710066
WhatsApp: +44 (0) 7716 503156
Email: support@diamedica.co.uk
Web: www.diamedica.co.uk
Alphamed Consulting Ltd, Knock, Barnaderg, Tuam,
Co. Galway, H54 W220
Revision D 17/02/2022DCN-0142
1639

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 2of 25
Read this page first
INTENDED USE
The DPA02 facilitates the administration of inhalational anaesthesia and respiratory support in
difficult environments and low resource settings. The units are contained in a protective Peli-
case and are portable for deployment to field operations or humanitarian emergency situations.
This device is suitable for use in hospital settings with limited resources or in any field or
outreach locations and is suitable for adult and paediatric patients.
The DPA Anaesthesia Series is not intended for use in The EU (With the exception of
supervised training by qualified personnel)
FOREWORD
This manual is intended to provide guidance on the function, performance, and user
maintenance of the DPA02 Anaesthesia System. The information given inthis manual is correct
at the date of publication.
The policy of Diamedica (UK) Ltd is to continuously improve its products. Changes may be made
to this manual without notice being given.
Users of the DPA02 Anaesthesia System must read, understand, and follow the guidance
given in this manual before using the system.
THE NEED FOR PATIENT MONITORING. WARNING
The DPA02 Anaesthesia System delivers mixtures of gases and vapours which could cause injury
or death to the patient. The effect of anaesthesia drugs on individual patients can vary so that
“typical” device settings for concentrations delivered to the patient do not necessarily ensure
patient safety.
The DPA, Diamedica Portable Anaesthesia Systems are designed for use in remote areas with
limited logistical support and emergency situations where ideal medical conditions are unlikely.
The ultimate responsibility for patient or procedure contraindication lies with the anaesthetist,
and will be situation dependent.
Medical conditions which contraindicate the use of a DPA Series Portable Anaesthesia Systems,
and its associated applications include any medical conditions which may contraindicate the
medical procedure itself.
The DPA series does not include patient monitoring for ETCO2 FIO2, Patient airway pressure,
expired volume, or PEEP. It is the responsibility of the clinician in charge to ensure suitable
monitoring is in place for the patient and procedure being performed and in the environment in
which it is being completed.

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 3of 25
Daily set up and test instructions should be successfully carried out to ensure that the DPA
Series of anaesthetic machines are in operating condition. If any parameter or test is found to
deviate from the instructions the machine should not be used, until the issue is resolved.
The Diamedica Portable Anaesthesia Series utilizes atmospheric air within the delivered mixture
to the patient it is therefore recommended, particularly in areas at risk of atmospheric
contamination that a single use bacteria filter is used within the patient circuit. HME and
breathing system filters should be medically compliant with recognized standards for use within
the region of operation.
It is essential that the patient’s respiration and cardiovascular status are frequently checked by
the anaesthetist.
The anaesthetist is ultimately responsible for patient safety and should always have a
secondary means of maintaining patient safety.
Observations of the patient must take precedence over machine settings in judging the
condition of the patient.
If ether is the only volatile agent available, it must be vaporised in a different vaporiser.
The Diamedica Portable Anaesthesia systems are transportable devices. The vaporiser must be
emptied of agent (Refer to section 11) and secured within the case prior to transportation.
Drawover anaesthesia is contraindicated for patients below 10kg, for these patients the
machine should be used in continuous flow (see section 9).
This User Manual must be stored near the product, protected from anything, which could
compromise its integrity and legibility.
NO MODIFICATION OF THIS EQUIPMENT IS PERMITTED.
The system is only intended to be used by Qualified Anaesthetists.
Method(s) of sterilization
The DPA series is a non-sterile device and is not intended to be sterilized by the user.
Suitability for use in an OXYGEN RICH ENVIRONMENT
Intended for use in an Oxygen rich environment.

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 4of 25
THE DPA 02 MANUAL
1. INTRODUCTION
2. SPECIFICATIONS
3. CLEANING, GENERAL MAINTENANCE AND DISPOSAL
4. THE COMPONENT PARTS OF THE DPA 02
5. CONTROL AND OPERATION
6. SUPPLEMENTRY OXYGEN SOURCES
7. TEST PROCEDURE BEFORE USE
8. USE OF THE DPA 02 ON ADULTS
9. USE ON PAEDIATRIC PATIENTS
10. PEEP (Positive End Expiratory Pressure)
11. FREQUENTLY ASKED QUESTIONS
12. SYMBOLS GLOSSARY

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 5of 25
1. INTRODUCTION
In many parts of the world anaesthetics are administered in situations far removed from those
found in modern, well equipped hospitals in wealthy countries. There may,for example, be no
oxygen, electricity, or technical support. In these circumstances, the latest sophisticated
anaesthetic machines with their delicate monitoring devices are unable to function and are
rapidly consigned to the graveyard of anaesthetic equipment which litters the developing world.
Anaesthetists working in such environments need equipment which goes beyond the standards
of those required for hospitals in rich countries. Equipment is needed that has been specifically
designed to meet the additional requirements of harsh environmental conditions and limited
infrastructure and that will continue to function in those prevailing conditions. When advice has
been sought from anaesthetists working in these areas the following properties have been most
frequently requested:
The anaesthetic machine should be:
1. Easy to understand and operate.
2. Robust and not easily damaged
3. Inexpensive to purchase and economical to run.
4. Maintained using locally available skills.
5. Safe to use in the absence of expensive electronic monitoring equipment.
6. Versatile, so that the same machine can be used on any size of patient, with a
variety of volatile agents, in either draw over or continuous mode.
7. Able to continue operating without interruption in the absence of oxygen or
electricity.
8. Be resilient to unstable or intermittent mains power supplies.
The DPA series of anaesthetic machines has been developed to meet these requirements and
the needs of anaesthetists working in difficult environments.
The DPA 02 is a free-standing anaesthetic machine in a transport case suitable to be carried by
a single individual. It has been specifically designed to facilitate the administration of
inhalational anaesthesia in difficult environments. It is easy to understand and operate,
economical to run and can be maintained and serviced using locally available skills. Above all,
it does not require compressed gases or electricity.
This manual has been prepared to provide practical guidance for those using the DPA series. It
should only be operated by experienced anaesthetists who have received specific training in
its use and are fully competent in its operation.

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 6of 25
2. SPECIFICATIONS
The DPA 02 is suitable for adult and paediatric use. The specifications are listed
below:
Component / Feature Specification
Dimensions (Closed case)
Height
17cm
Width
47cm
Depth
35cm
Weight
9.8kg
Operating
Environment
Temperature
Humidity
Altitude
5 - 40ᵒC
35% - 90% H
79 – 106 kpa
Storage
Environment
Temperature
Humidity
Altitude
-10 - +45ᵒC
15% - 93% H
79 – 106 kpa
Maximum operational altitude
< 2000m
Oxygen concentrator
Regulated external gas supply (Cylinder or wall)
0.5 Bar Min.
5 Bar Max.
PEEP; circuit dependent
0 – 20 cm H2O
Vaporiser
Low inspiration resistance
<0.6kpa
Suitable for Drawover
and continuous flow
Yes
Anaesthetic agent
Isoflurane / Halothane or
Sevoflurane
Capacity
150ml
Agent concentration range. **
ISO / HAL 0 – 5%
SEV 0 – 8%
** Delivered concentration accurate within ±20 % of set value for concentrations (volume
fraction) greater than 1 % and ±50 % of set value for concentrations of 1 % or below.

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 7of 25
3. CLEANING, GENERAL MAINTENANCE AND DISPOSAL
The anaesthesia machine usage should be clearly logged and recorded to assist
maintenance and cleaning activities.
This can be done in a format suitable to the user or in a format as shown below.
Date
Task
① Patient use
② Maintenance
③ Cleaning
Time on
Time off
Agent
Comments / Completed tasks
Suggested usage log for DPA series
The anaesthesia unit and case should be cleaned daily by wiping down with a damp cloth, care
should be taken to ensure that any sharps have been removed and disposed of safely before
this is done.
Ensure unit is dry free from moisture after wiping.
The ambient air intake grille should be
inspected for any particulate matter which
should be removed if present.
Patient safety is the primary concern of the Clinician and infection control is critical to ensuring
the safety of medical procedures. Appropriate cleaning and disinfection is essential after each
patient usage.
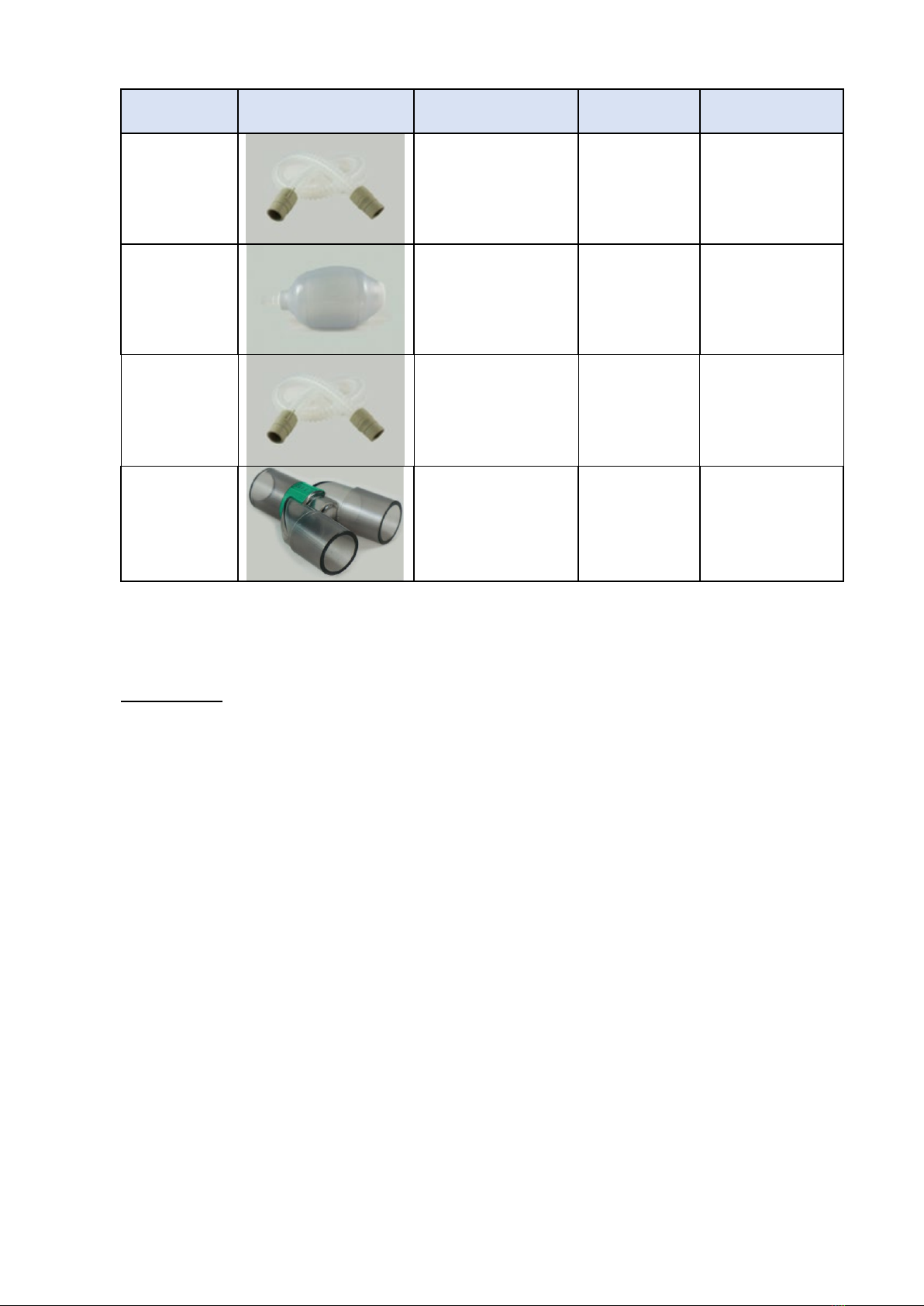
(i) Breathing circuit
Each DPA is supplied with a reusable breathing circuit. as these items may come in contact
with the patient and can therefore potentially pass infectious agents from one patient to
another if used improperly, the reusable breathing tubing and patient valve provided with the
anaesthesia machine should be cleaned and disinfected according to your hospital’s infection
control procedures. If no bacteria filter is used, then the entire circuit should be cleaned and
disinfected after each patient or after any contamination event involving the breakdown of
the completed circuit. Refer to table below.
DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 8of 25
Component
Image
cleaning
requirements
Frequency
Comments
Patient limb
Wash in bleach
solution, rinse and
dry in line with
hospital’s infection
control procedures
Weekly / after
emergency
field usage
Examine for
damage, replace
if necessary.
Self inflating
bag
Wash in bleach
solution, rinse and
dry in line with
hospital’s infection
control procedures
Weekly / after
emergency
field usage
Examine for
damage, replace
if necessary.
Limb to self-
inflating bag
Wash in bleach
solution, rinse and
dry in line with
hospital’s infection
control procedures
Weekly / after
emergency
field usage
Examine for
damage, replace
if necessary.
Patient ‘Y’
Piece
Wash in bleach
solution, rinse and
dry in line with
hospital’s infection
control procedures
Weekly / after
emergency
field usage
Examine for
damage, replace
if necessary.
(ii) Any bacteria filters and other single-use items provided should be discarded after one use
since they are not designed to be reprocessed.
(iii) Vaporiser
Halothane decomposes over time causing the release of halides, which can corrode metal
components, particularly in the presence of moisture. For this reason, a stabilizing
agent, thymol, is added to prevent decomposition. Since thymol does not volatilize along with
halothane, it can accumulate in the vaporizer, making the control lever stiff.
If the control lever is stiff, it may be the result of accumulated thymol. You can perform the
following to try to loosen the lever:
1. Remove the vaporiser from the stand and set to zero.
2. Turn it upside down, and shake it vigorously followed by moving the lever until it becomes
loose.
3. When the lever loosens, it should be drained and rinsed with fresh agent.
4. Attach the vaporiser to the control panel and fill with fresh halothane.
The vaporiser should not require recalibration. Any Operational calibration should only be done
following consultation with manufacturer.

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 9of 25
Accessories and spares
The patient circuit tubing is Non-conducting (Applied Part). DO NOT replace with
conducting/anti-static tubing.
All accessories used with the DPA-02 must:
•Be oxygen compatible,
•Be biocompatible,
A full list of available spares is available by contacting Diamedica – support@diamedica.co.uk
Technical data enquiries
For all technical, performance or component related enquiries please contact Diamedica -
support@diamedica.co.uk
Method for disposing of the device
If the product is returned to the manufacturer at the end of its life the company will ensure
disposal in line with the relevant disposal regulations

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 10 of 25
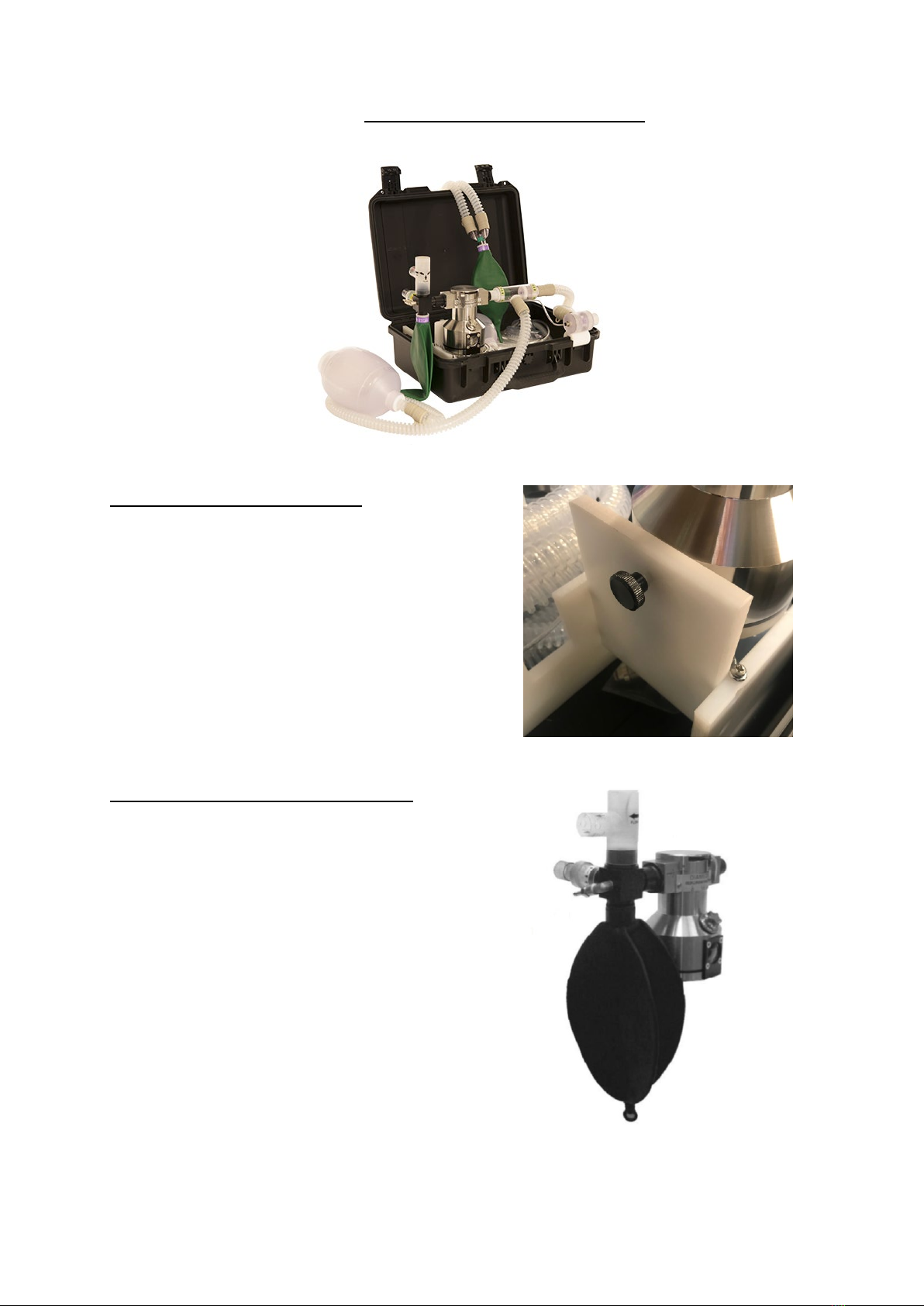
4. THE COMPONENT PARTS OF THE DPA 02
The Diamedica Portable Anaesthetic system DPA-02 has four principal components.
•Protective Peli case.
•Reservoir.
•vaporiser.
•Breathing system.
These are configured as follows:
Peli case.
This carrying case features a soft-grip handle
and two easy-open press-and-pull latches.
The case protects against the elements - it is
dustproof, crushproof, and waterproof, rated
IP67 when latched shut for transportation.
Ensure labelling is not damaged or removed
and always store in an upright condition.
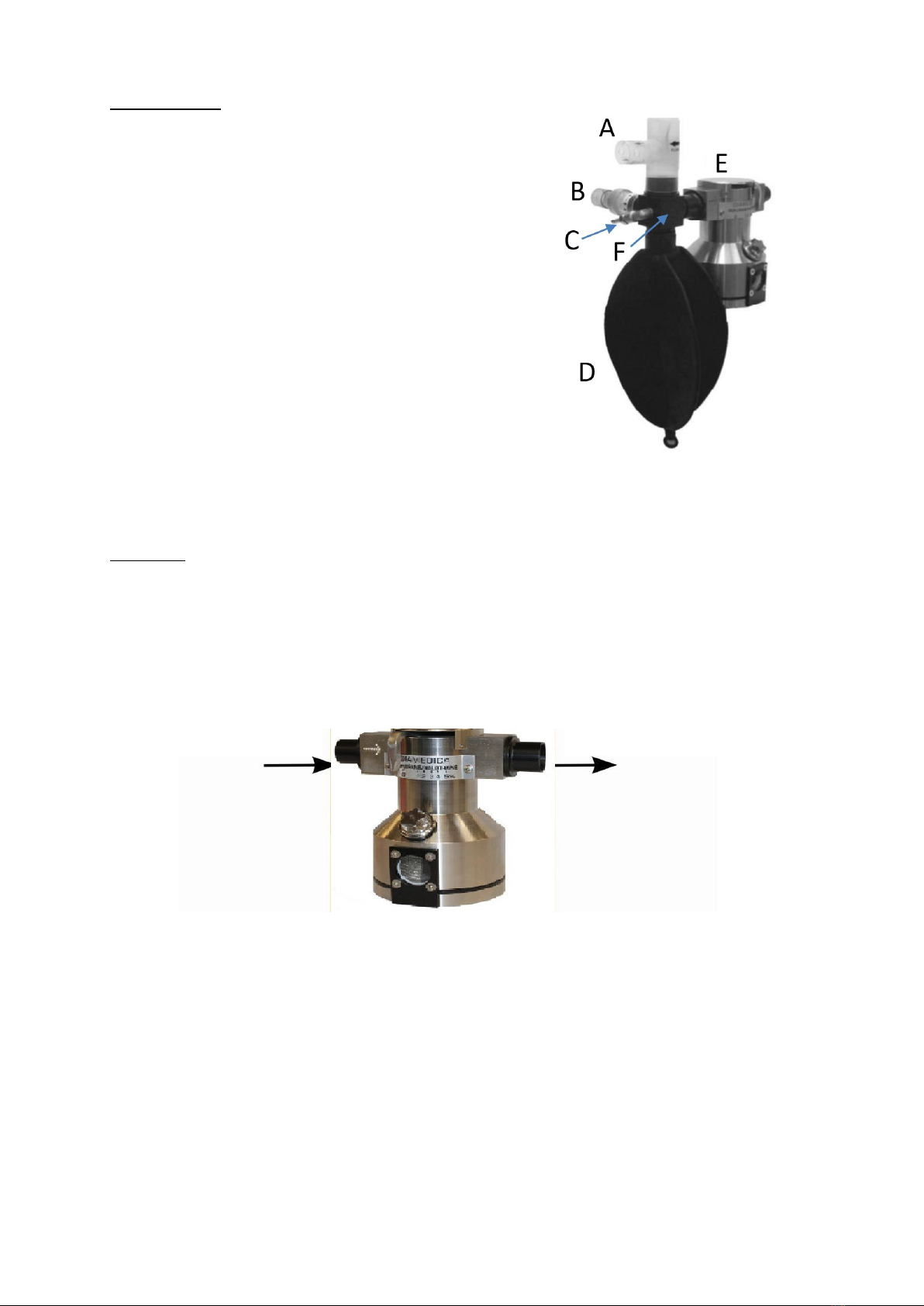
DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 11 of 25
Flow
Flow
The Reservoir.
A. The pressure relief valve with outlet pressure set
at 7.5cm water.
B. The air entry one way valve with arrows
indicating direction of air flow.
C. The oxygen supplementation port (metallic nozzle).
D. The 2-litre reservoir bag.
E. Vaporiser.
F. Connecting block.
Vaporiser.
Before a volatile anaesthetic agent can be administered to a patient it must first be vaporised. A
carrier gas containing oxygen passes through the chamber of a vaporiser where vaporisation
occurs, and the resulting mixture is delivered to the patient.
Pressure Gradient
In order for the carrier gas to pass through the vaporiser there must be a pressure gradient
between entry and exit ports of the vaporiser. The carrier gas must therefore either be PUSHED
through by positive pressure from upstream or DRAWN through by negative pressure from
downstream.
By contrast in DRAWOVER anaesthesia the carrier gas is DRAWN over the vaporiser by negative
pressure generated by the patient’s inspiration. The great advantage of draw over anaesthesia
is that it can still be administered EVEN IF THE OXYGEN SUPPLY FAILS. In this situation room
air, containing 21% oxygen, can be used as the carrier gas for the volatile agent which is
supplemented with oxygen if available.

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 12 of 25
PATIENT VALVE
The DPA 02 can function as a continuous flow machine when gases are provided by an Oxygen
concentrator or an auxiliary source. However, if these sources fail the system will default to a
drawover machine in order for anaesthesia to continue safely.
This conversion happens automatically in the event of gas failure or Drawover can be used in
order to conserve both oxygen and anaesthetic agent. This is described further in later sections
of the manual.
The Diamedica vaporisers output is consistent in both modes, the output from other Drawover
vaporisers may not be suitable for both these modes.
The flow capabilities of the draw-over vaporizer meet the requirements of ISO 18835:2015 and
can operate consistently up to an intermittent peak inspiratory draw of 35 L/min
Typical capabilities @ 6l/min are represented in the graphs below.
1% Continuous
4% Continuous
1% IPPV
4% IPPV

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 13 of 25
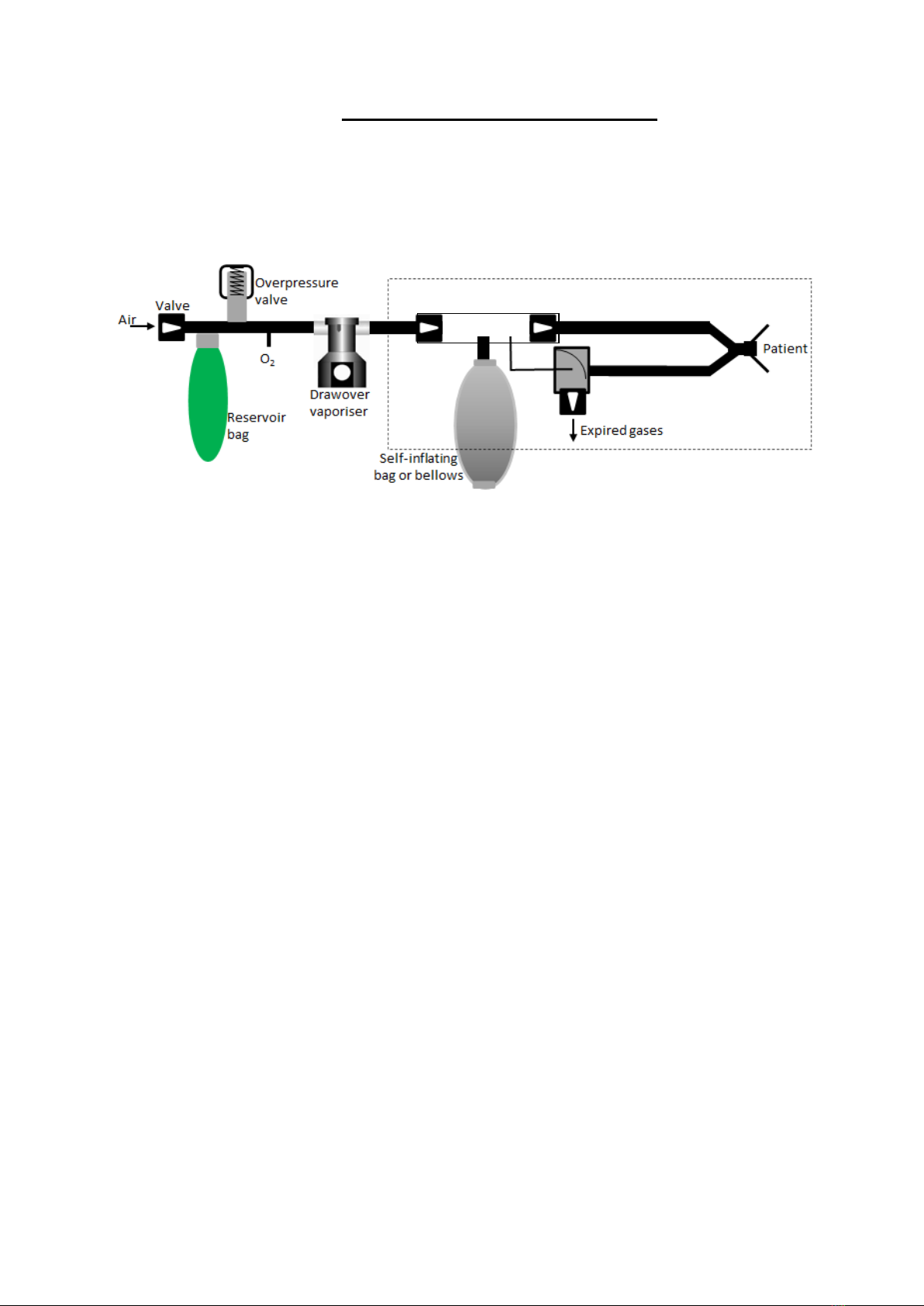
The breathing system.
1. The Inspiratory valve (A). This consists
of a one-way valve at either end of a clear
cylindrical tube to control the direction of
flow. The side wall of the tube has 2 further
connections as
2. A Self-inflating bag (B) is connected to a
connection port on the side wall of the
inspiratory valve. This is used to induce flow
across the vaporiser and on to the patient
when not breathing spontaneously.
3. The Expiratory valve (C). PEEP valve can
be affixed to the vent port of this valve –
Refer to section 10.
4. Pressure stabilization tube (D) between
inspiratory and expiratory valves. This
closes the expiratory valve during the
inspiratory phase to allow the lungs to fill
5. 22mm silicon respiratory tubing (E1& E2)
is connected to the Inspiratory and
expiratory valves with the opposite ends
joined together with a standard ‘Y’ piece
and 1 litre bag to act as a test lung.
Gas Circuit diagram
A
B
C
E1
D
E2
A length of standard respiratory
tubing (supplied) for scavenging
of expired gases away from the
operational area to be attached
here.
DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 14 of 25
5.
CONTROL AND OPERATION
The Diamedica Portable Anaesthetic machine DPA 02 must be assembled as follows.
Position and secure the vaporiser
(1) Remove the vaporiser from the storage
location and place it on the wire grill.
(2) Secure the back of the vaporiser to the
upright partition of the stand using the
supplied screw.
Assemble and attach the reservoir block
(1) Take reservoir block and affix the reservoir bag to
the connection port on the opposite side to the
pressure relief valve.
(2) Attach the assembled reservoir block to the
input port on the left side of the vaporiser.
(3) If using a supplementary Oxygen supply connect
this to the supply port on the end of the block with
the other end connected to the regulator if using a
cylinder or directly to the supply port of an oxygen
concentrator. Refer to Section 6.
F

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 15 of 25
Assembly of breathing system.
1. Attach the Inspiratory valve to the vaporiser outlet ensuring the direction of flow is correct
(Arrows pointing away from the vaporiser).
2. Attach the self-inflating bag and tube to the port
between the two valves on the inspiratory valve.
3. Fix the expiratory valve to the case
4. Assemble the two corrugated tubes to the Y piece
and fit the 1 litre test lung
NOTE
Test lung to be replaced with patient interface
once correct circuit set-up is confirmed.
5. Attach one off the tubes to the outlet of the
inspiratory valve and the other tube to the
Expiratory valve.
To test the assembly:
Test the integrity of the system using the self-inflating bag. The test lung (1 litre green reservoir
bag) should fill and hold pressure as the Self inflating bag is compressed and release pressure
when the Self inflating bag is released. The reservoir bag will indicate flow/breathes when
connected to a supplementary oxygen supply, as it will inflate between breathes and release on
the inspiratory cycle.
DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 16 of 25
The vaporisers are designed to be used with specific anaesthetic agents as clearly labelled.
Where dual agents are permitted it is the users’ responsibility to ensure that only same
agent is added to the vaporiser or that the chamber is emptied before filling with fresh
agent. In the case of single agent vaporisers, the filler tubes are agent specific. The fittings
on the vaporiser and the collar of the bottles are specific to the agent too. This precaution
is built into the design to prevent mixing of the anaesthetic agents.
Vaporisers must not be overfilled or underfilled to prevent failure of the vaporizer systems.
Ensure that only a single agent as specified on the scale is used.

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 17 of 25
6. SUPPLEMENTRY OXYGEN SOURCES
The DPA series will accept supplemental oxygen from an oxygen concentrator.
It will also accept supplemental oxygen from a regulated cylinder (the regulator flowmeter
below is supplied), or from a regulated central oxygen supply.
Insert supplied Oxygen tube onto barbed connection as shown above, ensuring
that connection is fully inserted
The opposite end of the oxygen tube must
be connected to the barbed connector on
the supplied regulator (as shown) or
directly to the relevant output port of an
oxygen concentrator.

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 18 of 25
7. TEST PROCEDURE BEFORE USE
Confirm vaporiser contains the correct volatile agent and that concentration lever moves
freely. Refill vaporiser if required.
Turn on oxygen supply if available. Always ensure that the cylinder has sufficient content
prior to use.
Ensure gas scavenging tube is connected to the 30mm outlet of the Expiratory valve and
that tail end of tube is suitably positioned away from the operational area.
Test anaesthetic circuit. Attach a one litre reservoir bag to the end of the patient circuit to
act as test lung. Compress the self-inflating bag to demonstrate expansion of test lung
and simultaneous movements of reservoir bag.
Remove test bag and affix patient interface (and filter – recommended)
Unit is now ready for use
DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 19 of 25
8. USE OF THE DPA 02 ON ADULTS
In Adult patients (and paediatric patients above 10 kg) the standard Y piece dual
limb circuit is used (Refer to Section 5 – Control and operation).
Correct vaporiser settings for induction and maintenance of anaesthetised stated
are clinical decisions based on patient evaluation and ongoing monitoring.
Inspiratory valve
Expiratory
valve

DPA 02 MANUAL ENGLISH © Copyright Diamedica (UK) Ltd 2022 Page 20 of 25
9. USE ON PAEDIATRIC PATIENTS
In patients less than 10 kg the continuous flow paediatric circuit (Mapleson F – Ayres T piece)
should be used with a flowrate of at least 3 times the patients minute volume.
Ayres ‘T’ Piece paediatric circuit
The circuit can be connected directly to the inspiratory valve section as below. It is
recommended that this circuit should be used with a minimum fresh gas flow from
concentrator or cylinder of at least 3 x the patient’s minute volume.
The self-inflating bag and inspiratory valve can stay in position.
Other manuals for DPA 02
1
Table of contents
Other Diamedica Medical Equipment manuals