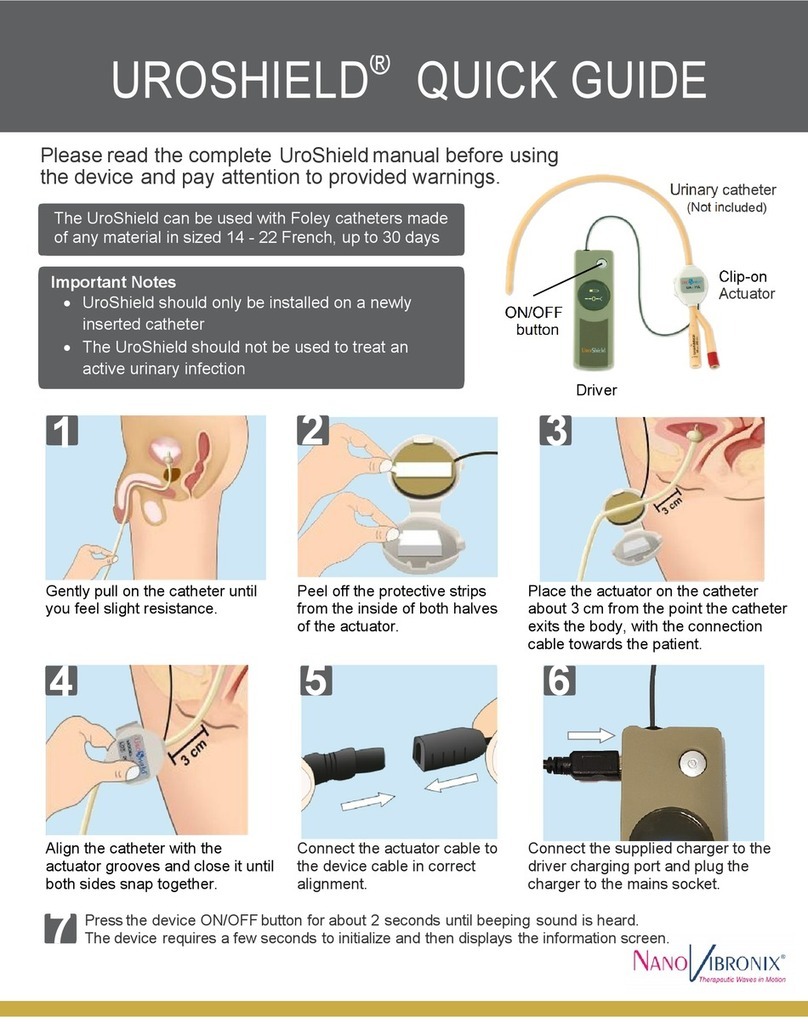
Wound Shield user manual
NanoVibronix Page 4 of 26
General Information
Introduction to ultrasound
Ultrasound is a form of acoustical vibration occurring at frequencies above the 20 kHz perception limit of
the human ear. Ultrasound therapy effectiveness depends on transmitting pressure and displacement
waves through body tissues. Since the body is actually composed of a variety of tissue types, the
penetration depth will depend on the thickness of each tissue in the pathway of the ultrasound beam.
Therapeutic ultrasound is produced through a reverse piezoelectric effect. Electric signals produced by
the driver are delivered to an electrode that is in contact with the piezo electric element. The signal
applied to the piezo element surface produces mechanical vibrations, or the so-called reverse piezoelectric
effect.
Ultrasonic power is expressed in watts (W), or watts per square centimetre (W/cm2). Average intensity
(W/cm2) is obtained by measuring the total output of the applicator (in watts) and then divided by the size
of the effective radiating area of the applicator.
High frequency waves (Megahertz range) are absorbed rapidly with consequent reduction in penetration.
Conversely, lower frequencies (kilohertz range) support wave penetration and may lead to greater energy
deposition.
The WoundShield Device
It is known in the art, that ultrasound is beneficial in all wound healing phases:
inflammatory - it enhances the degranulation of mast cells resulting in the
release of histamine and other mediators that attract fibroblasts and endothelial
cells to the injured area; proliferative - stimulates fibroblast migration and
proliferation to secrete collagen, improving tensile strength of the healing
connective tissues; Epithelialization - stimulates the release of growth factors
needed to regenerate epithelial cells; Maturation or Remodeling - affects the
collagen extensibility and enzyme activity and therefore also improves tensile
strength of the healing tissue