3
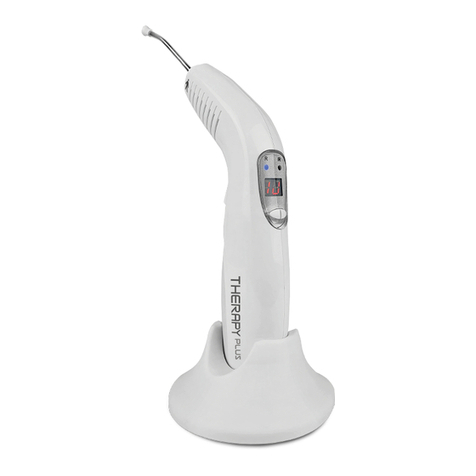
Therapy EC is a high-technology equipment which meets the most recent national manufacturing
standards required by ANVISA - Agência Nacional de Vigilância Sanitária [National Agency for San-
itary Vigilance].
Therapy EC was created to be used by medical and dental professionals. This professional must
be able to apply the techniques related to the product. Its inappropriate use may bring irreversible
injuries.
EQUIPMENT FUNCTIONS
Therapy EC releases red and infrared laser.
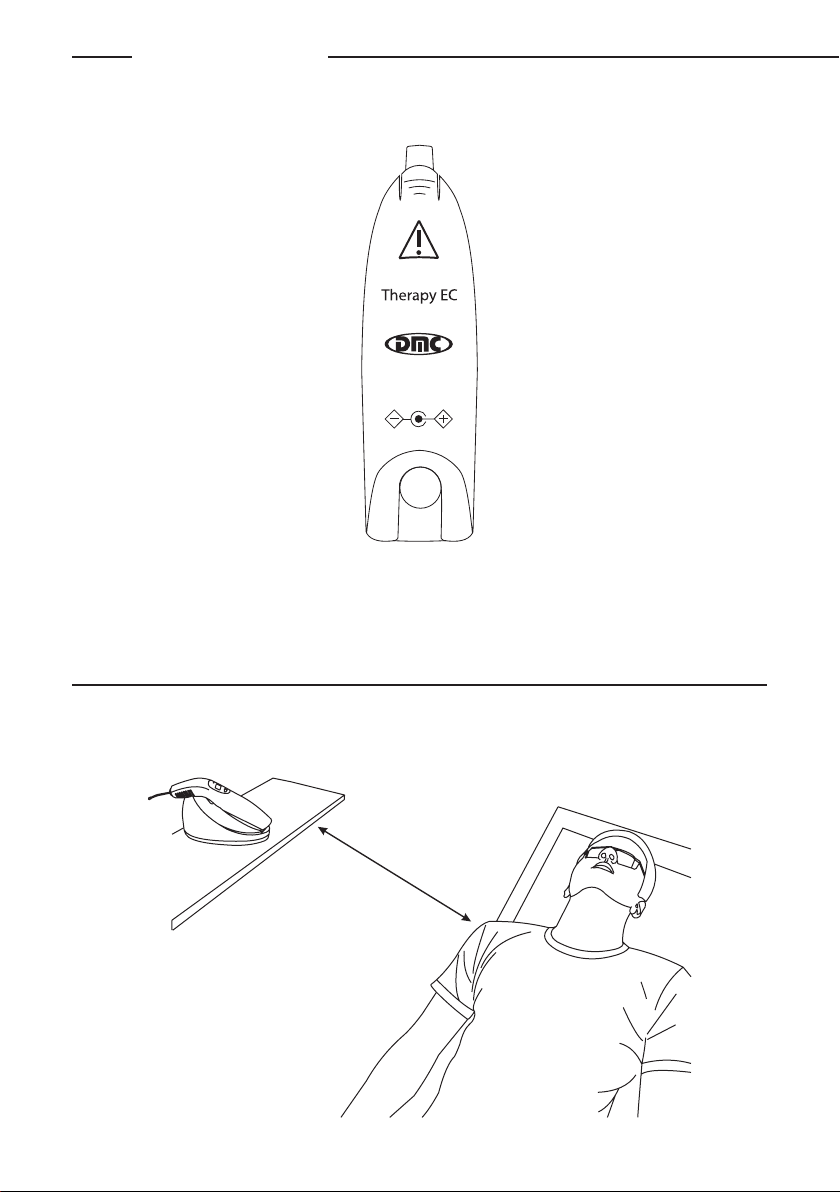
EQUIPMENT WORKING
By means of a small display and three keys, the operator views and performs all settings and features
of the equipment.
INDICATIONS
LASER THERAPY
Soft Tissue Recovery (Red Laser): Aphthae and traumatic ulcer, oral manifestation of systemic
diseases, lichen planus, lupus erythematosus, pemphigus vulgaris, gingival hyperplasia (diabetes),
cheilitis angular, gingivitis, postoperative, ATM dysfunction, acne, healing, combats free radicals
(Ilib Technique), lower limb ulcers, venous ulcers, arterial ulcers, diabetic ulcers, cutaneous ulcers
of different etiologies, venous stasis ulcer, skin scores, vascular microcurrent diabetes, post-surgery,
contact dermatitis, burns, pressure ulcer, diabetic ulcer and varicose ulcer.
Bone Tissue Recovery (Infrared Laser): Orthodontia, implantodonty, periodontitis, extraction,
traumatic injury, biostimulation of bones, Left Femur Fracture and Middle Third Closed Fracture,
Faster Consolidation of Fractured Bone, Cartilage and Bone Regeneration and Sjögren’s Syndrome.
Dental Tissue Recovery (Red and Infrared Laser): sensitivity following cavity preparation, sensitivity
following scaling, amelogenesis imperfecta, dentine hypersensibility, decubitus ulcers and acute in-
flammation.
Nerve Recovery (Infrared Laser): Neuralgias, paresthesias, paralysis and pain syndrome, hand
articulation, arthritis, rheumatoid arthritis, biomodulation, neck pain, DMED pain, epicondylitis,
fasciitis plantar, fibromyalgia, gonarthrosis, rotator cuff injury, muscle injuries, cartilage injuries, pe-
ripheral nerve injuries, low back pain, osteoarthritis, facial paralysis, paresthesias, post-surgery, nerve
repair, carpal tunnel syndrome, myofascial pain syndrome, calcaneal tendinitis, patellar tendinitis
and tendinopathies.