3
Therapy Plus is a high-technology equipment which meets the most recent national manufacturing standards
required by ANVISA - Agência Nacional de Vigilância Sanitária [National Agency for Sanitary Vigilance].
Therapy Plus was developed to be used by doctors, nurses, dental surgeons, physiotherapists, acu-
puncturists, podiatrists and speech therapists. The professional must be qualified to apply the prod-
uct techniques. Improper use may lead to irreversible damage.
EQUIPMENT FUNCTIONS
Therapy Plus emits red and infrared laser light for the following purposes: tissue repair, inflammatory
process modulation and analgesia.
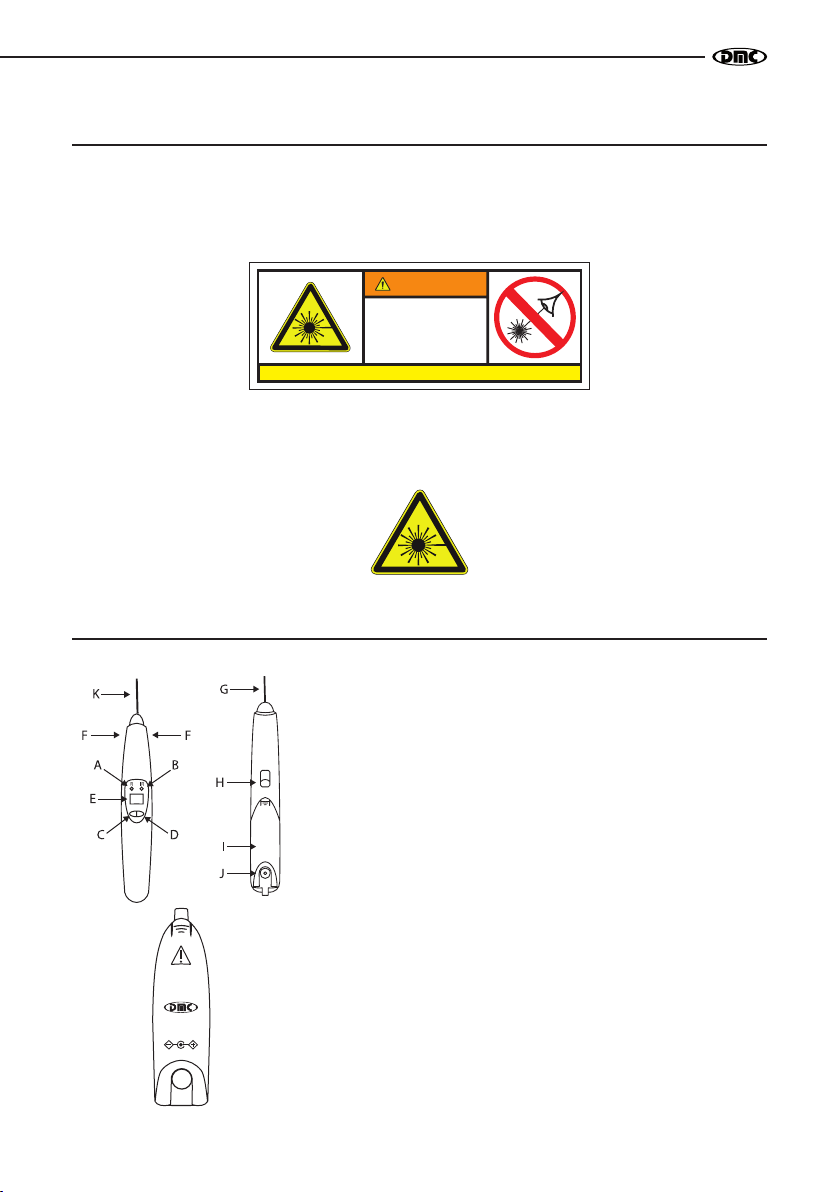
EQUIPMENT WORKING
By means of a small display and three keys, the operator views and performs all settings and features
of the equipment.
INDICATIONS
Therapy Plus has the following indications:
Red Laser: Aphthae and traumatic ulcer, oral manifestation of systemic diseases, lichen planus,
lupus erythematosus, pemphigus vulgaris, gingival hyperplasia (diabetes), cheilitis angular, gingivitis,
postoperative, ATM dysfunction, healing, lower limb ulcers, venous ulcers, arterial ulcers, diabetic
ulcers, cutaneous ulcers of different etiologies, venous stasis ulcer, skin scores, vascular microcurrent
diabetes, post-surgery, contact dermatitis, burns, pressure ulcer, diabetic ulcer and varicose ulcer,
Left Femur Fracture and Middle Third Closed Fracture, Faster Consolidation of Fractured Bone, sys-
temic laser therapy (ILIB technique).
Infrared Laser: Orthodontia, implantodonty, periodontitis, extraction, traumatic injury, biostimula-
tion of bones, Cartilage and Bone Regeneration and Sjögren’s Syndrome, neuralgias, paresthesias,
paralysis and pain syndrome, hand articulation, arthritis, rheumatoid arthritis, biomodulation, neck
pain, DMED pain, epicondylitis, fasciitis plantar, fibromyalgia, gonarthrosis, rotator cuff injury, mus-
cle injuries, cartilage injuries, peripheral nerve injuries, low back pain, osteoarthritis, facial paralysis,
paresthesias, post-surgery, nerve repair, carpal tunnel syndrome, myofascial pain syndrome, calcane-
al tendinitis, patellar tendinitis and tendinopathies.
Red and Infrared Laser: Sensitivity following cavity preparation, sensitivity following scaling,
imperfect amelogenesis, dentine hypersensibility, alveolitis, edema, xerostomia, pericoronitis, an-
esthesia, benign migratory glossitis, simple herpes and zoster herpes, decubitus ulcers and acute
inflammation.