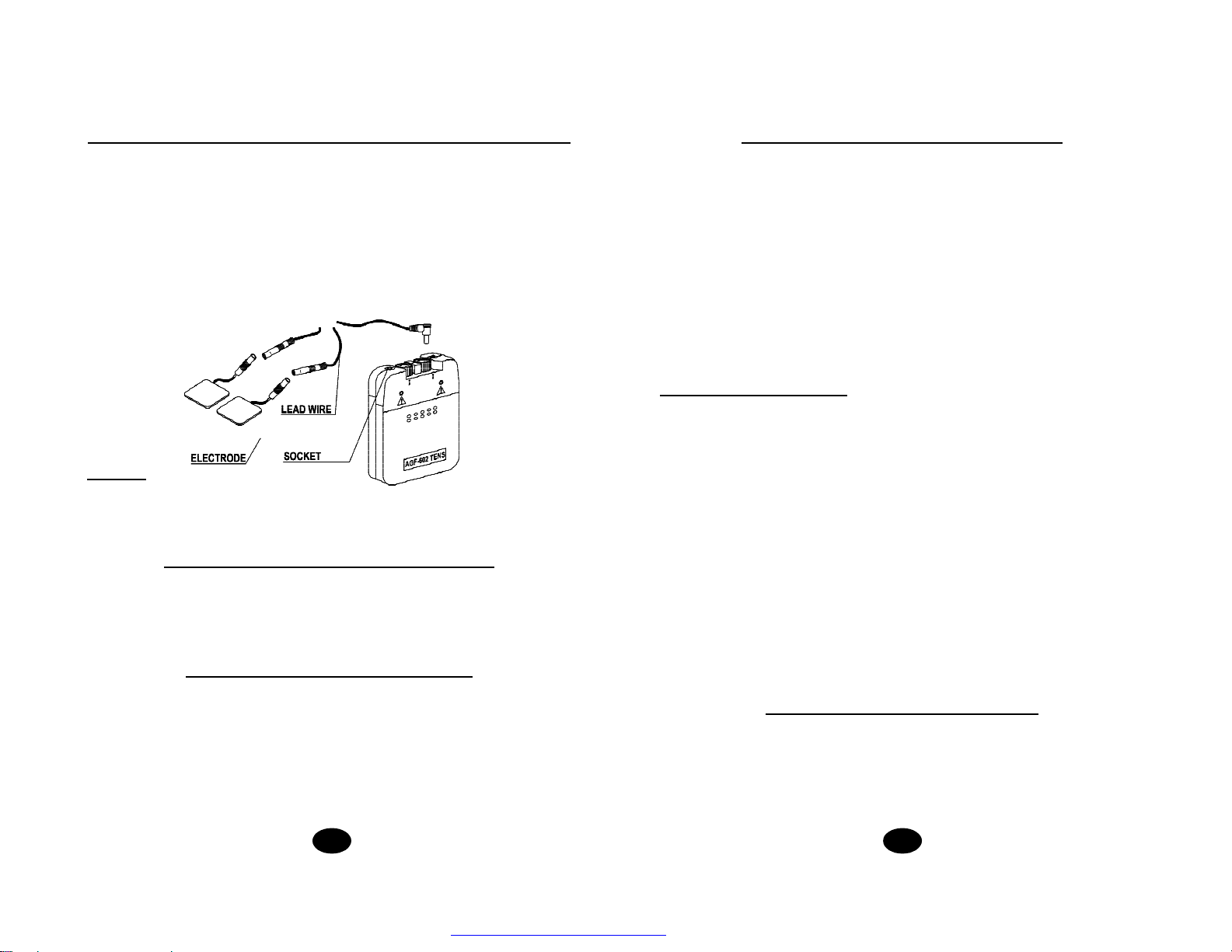
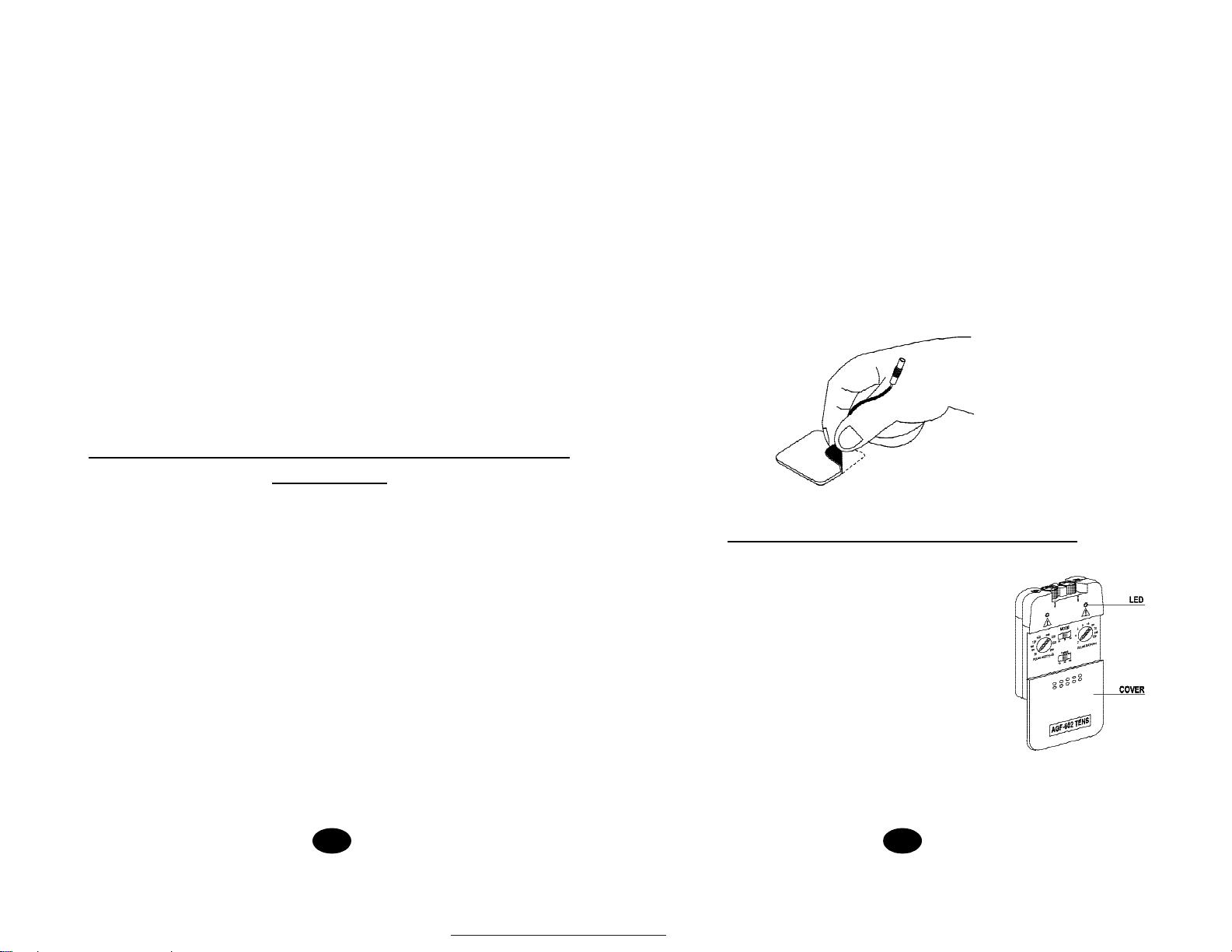
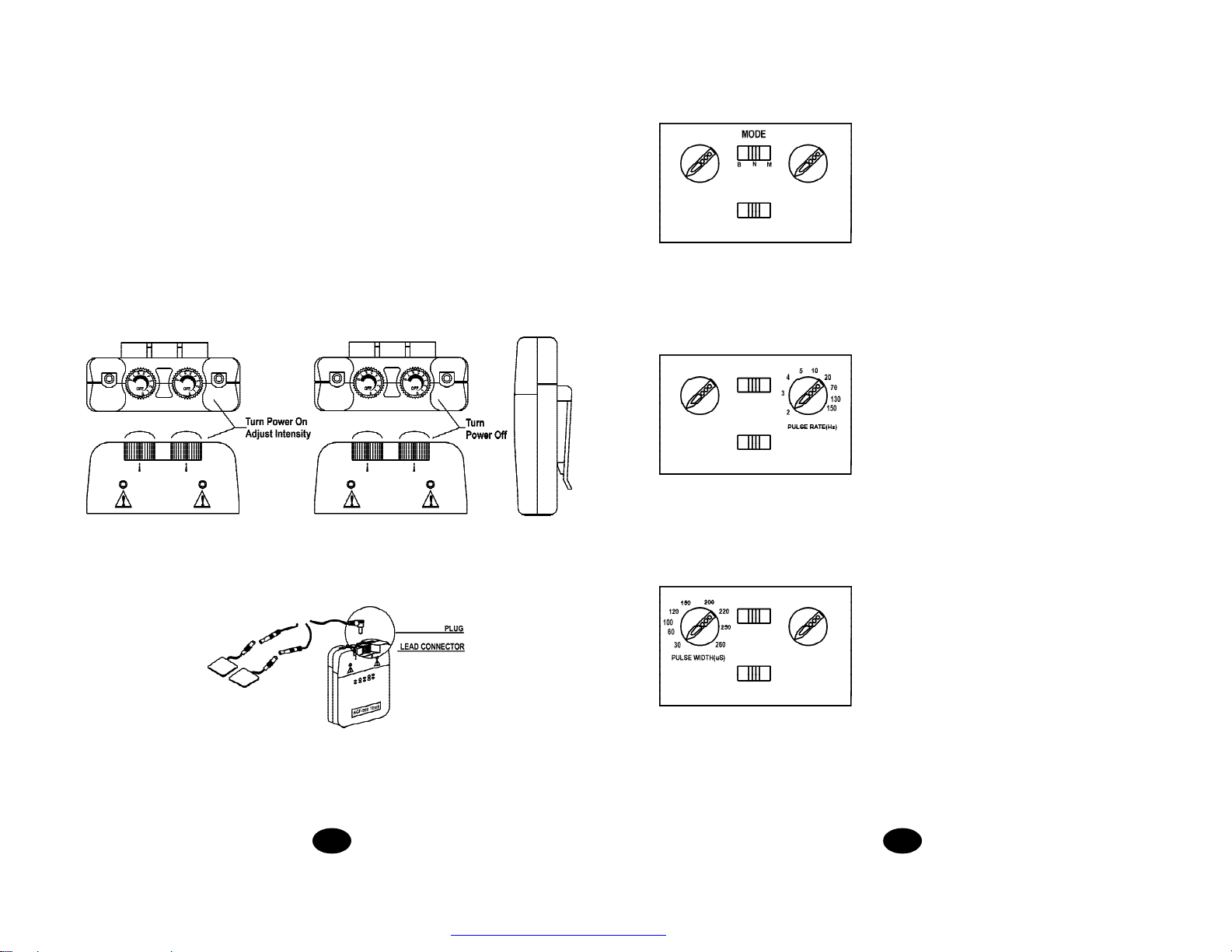
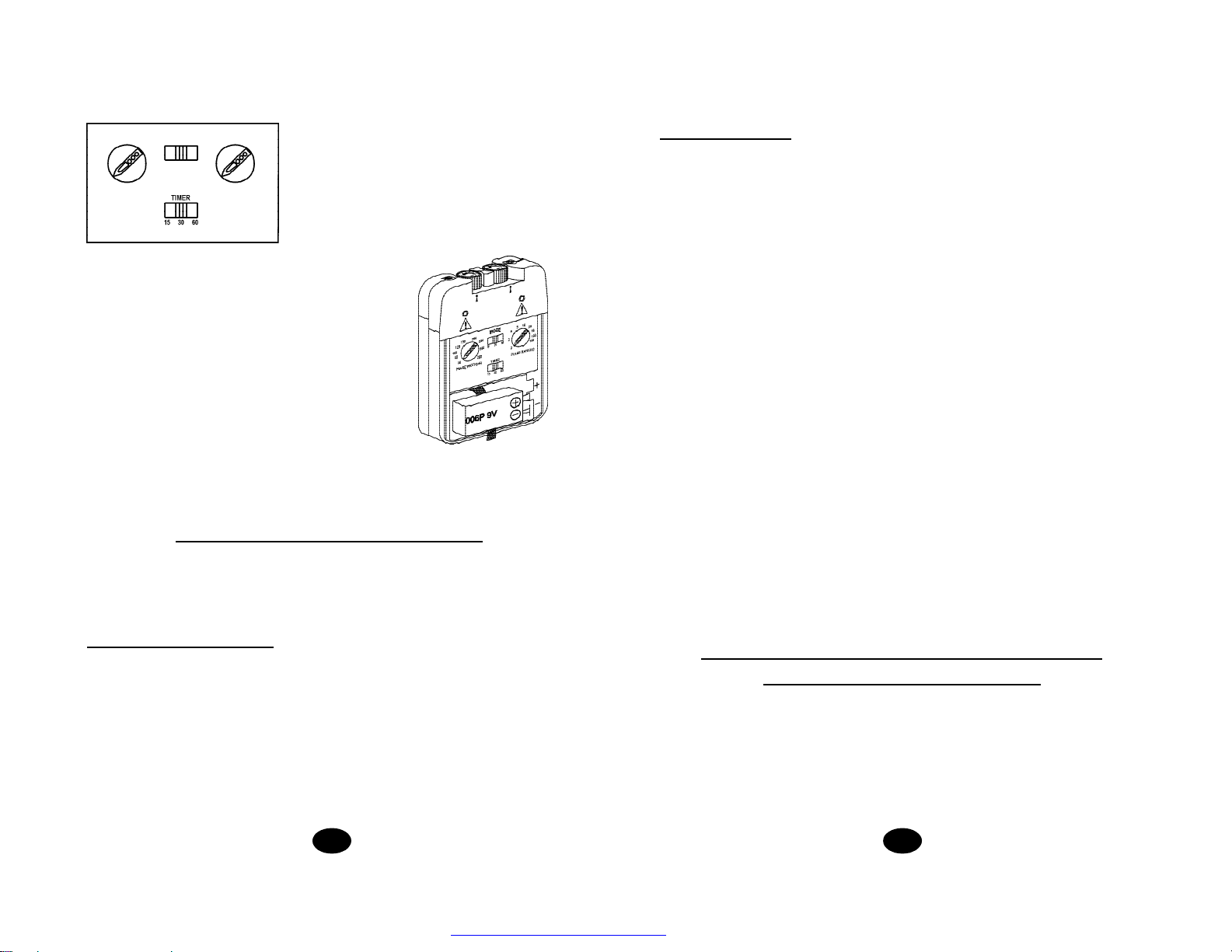
32
lected for therapy, and the type of pain. In many patients, the reduction or
elimination of pain lasts longer than the actual period of stimulation (sometimes
as much as three to four times longer). In others, pain is only modified while
stimulation actually occurs. You may discuss this with your physician or
therapist.
Chapter 2: CAUTIONS
1.Precautions:
Isolated cases of skin irritation may occur atthe site ofelectrode placement
following long-term application. Effectiveness is highlydependent upon patient
selection by a person qualified in the management of pain patients.
2.Contradictions:
TENS devices can affect the operation of demand type cardiac pacemakers.
TENS isnot recommended forpatients with known heart disease withoutphysi-
calevaluationofrisk.DonotuseTENSonthecarotidsinus(neck) region.Dono
apply TENS for undiagnosed pain syndromes until etiology is established. Do
notstimulateonthesitethatmaycausecurrenttoflowtranscerebrally -(through
thehead).
3.AdverseReactions
Possible allergic to gel, skin irritationand electrode burn are potential adverse
reactions.
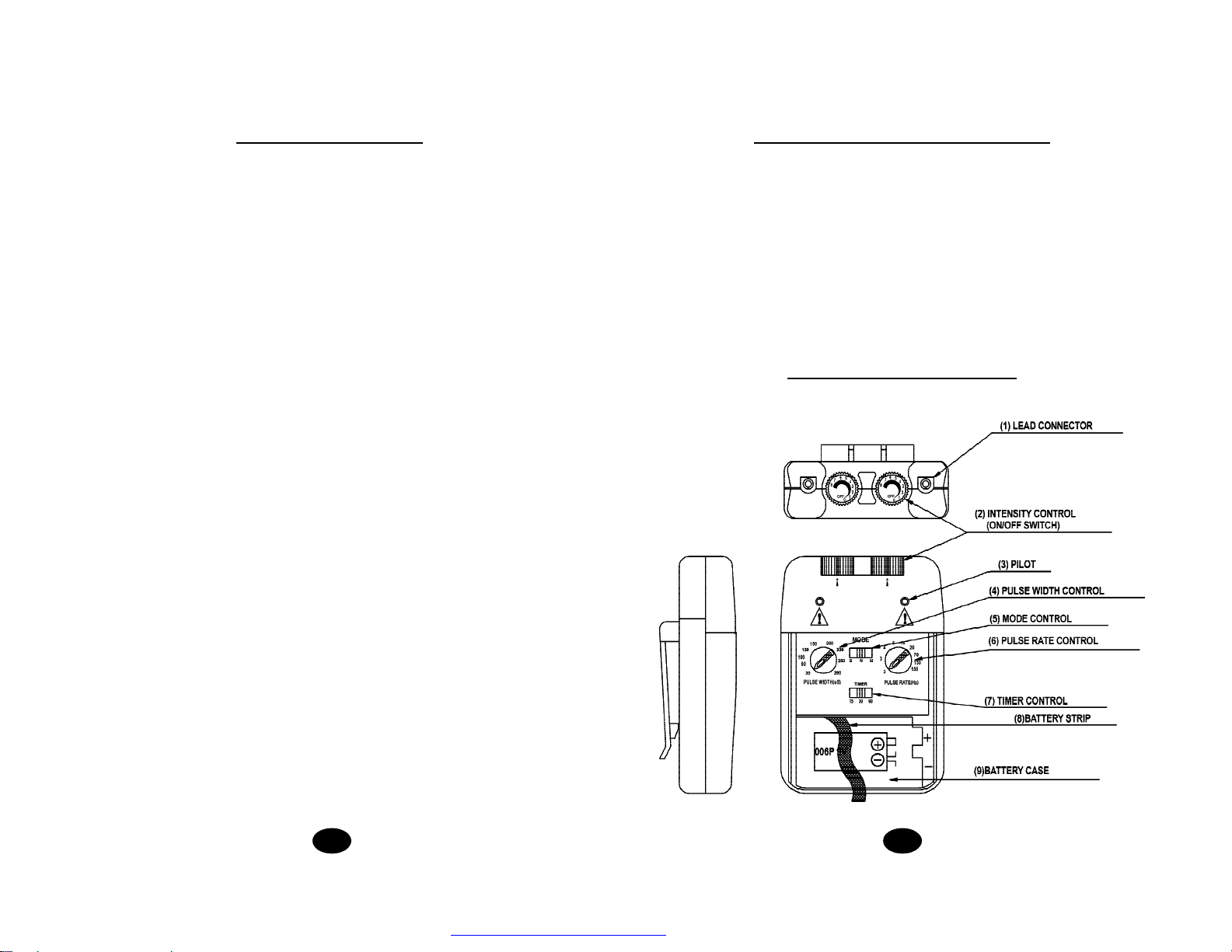
4.Read operation manual before use of TENS.
5.We emphasize that patient with an implanted electronic device (for example,a
pacemaker)shouldnotundergoTENStreatmentwithoutfirstconsultingadoctor.
Thesame appliesto patients with anymetallic implants.
6.If TENS therapy becomes ineffective or unpleasant, stimulation should be dis
continued until its use is reevaluated by the physician or therapist.
7.Avoid adjustingcontrols whileoperating machineryor vehicles.
8.Turn the T.E.N.S. off before applying or removing electrodes.
9.T.E.N.S. devices have no AP/APG protection.
Do not use it in the presence of explosive atmosphere and flammable mixture.
Chapter 1 : INTRODUCTION
EXPLANATION OF PAIN
Pain is a warning system and the body s method of telling us that something is
wrong. Pain is important; without it abnormal conditions may go undetected,
causing damage orinjury tovital parts of ourbodies.
Even though pain is a necessary warning signal of trauma or malfunction in the
body,naturemayhavegonetoofarinitsdesign. Asidefromitsvalueindiagnosis,
long-lasting persistent pain serves no useful purpose. Pain does not begin until
coded message travels to the brain where it is decoded, analyzed, and then re-
acted to. The pain message travels from the injured area along the small nerves
leading to the spinal cord. Here the message is switched to different nerves that
travel up the spinal cord to the brain. The pain message is then interpreted, re-
ferred back and the pain is felt.
EXPLANATION OF TENS
Transcutaneous ElectricalNerve Stimulation is a non-invasive, drug-free method
of controlling pain. TENS uses tiny electrical impulses sent through the skin to
nerves to modify your pain perception. TENS does not cure any physiological
problem;itonlyhelpscontrolthepain. TENSdoesnotworkforeveryone;however,
in most patients it is effective in reducing or eliminating the pain, allowing for a
return to normal activity.
HOW TENS WORKS
There is nothing “magic”about Transcutaneous Electrical Nerve Stimulation
(TENS). TENS is intended to be used to relieve pain. The TENS unit sends
comfortable impulses through the skin that stimulate the nerve (or nerves) in the
treatment area. Inmany cases,this stimulation willgreatly reduce or eliminatethe
pain sensation the patient feels. Pain relief varies by individual patient, mode se-
PDF created with FinePrint pdfFactorytrial version http://www.pdffactory.com