2
MUTE STATIC
POWER FAILURE
LOW PRESSURE
WEIGHT RANGE
1
MIN MAXMID
4 8
• Mattress only - For profiling beds, it is
essential that straps are secured around the
movable sections of the bed frame - damage
will be incurred when profiled if secured to
fixed parts of the frame (FIG 1).
• Cushion only: It is the responsibility of the
carer to ensure the chair is suitable for
product compatibility and the safety of the
patient. Position the cushion with the pipes at
the rear of the chair
• To avoid any risk of damage to the mattress/
cushion ensure there are no sharp objects
which may come into contact with it.
• Position the control unit by hanging the
hooks over the foot board. If there is no foot
board or the cushion is in use place the unit
on the floor with the front facing upwards.
Ensure the rear of the unit is not obstructed
by carpet, rugs etc. It is advisable to place the
unit on a firm surface (FIG 2).
• The mattress /cushion will start to inflate.
Inflation can take up to 40mins. Once inflated,
ensure the straps attaching the mattress
to the bed frame are secure and hold the
mattress in place. Secure sheets loosely
enough to ensure they do not interfere with
cell alternation.
CPR - mattress only
• Rapid deflation of the mattress may be required
for emergency treatment or system deflation.
The CPR pull tag is located at the foot end of
the mattress.
• Pull the CPR tag to disengage from the
mattress, once done the entire system will
rapidly deflate.
• To re-inflate, re attach the CPR tag (FIG 4).
• Wait for the mattress system to reach optimal
pressure prior to a return to normal use.
QUICK REFERENCE GUIDE P1 of 2
FIG 1. FIG 2.
FIG 3. FIG 4.
• Connect the mattress/cushion to the control
unit (FIG 5) ensuring the feed pipes do not
kink or become trapped between bed/chair
parts.
• Switch pump on by the ON/OFF switch (FIG 3).
• Mattresses - ensure that the CPR tag is
fully engaged within the mattress and all
connectors are lined up correctly (FIG 4).
TO BE USED IN CONJUNCTION WITH THE FULL INSTRUCTIONS FOR USE FOR THIS
PRODUCT. THIS QUICK GUIDE DOES NOT REPLACE THE FULL DOCUMENT.
THEIA Pump with mattress options and cushion
Overlay Mattress Air on Foam Mattress Dynamic Cushion
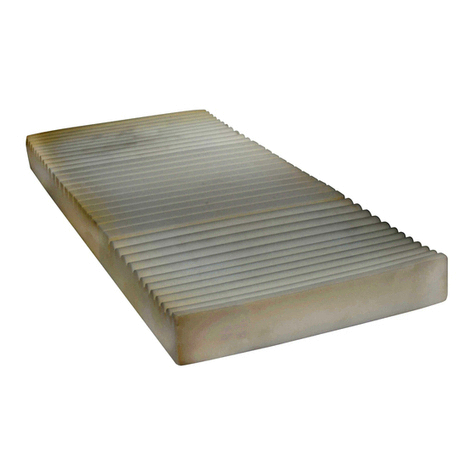
The mattress is intended to support a
single patient who is up to 180kg in weight
and 185cm in height. For those patients
of a very low weight, typically less than
40kg and a physical size less than 146cm,
clinical judgment is to be used to determine
suitability. A lower (or upper) age limit is not
defined as it depends on the physical size of
the patient in relation to the proportions of
the mattress and bed frame.
The mattress is intended to support a
single patient who is up to 180kg in weight
and 185cm in height. For those patients
of a very low weight, typically less than
40kg and a physical size less than 146cm,
clinical judgment is to be used to determine
suitability. A lower (or upper) age limit is not
defined as it depends on the physical size of
the patient in relation to the proportions of
the mattress and bed frame.
The cushion is intended to support a single
patient who is up to 120kg in weight. A
lower (or upper) age limit is not defined
as it depend on the physical size of the
patient in relation to the proportions of
the cushion and the platform. Clinical
judgement is to be used to determine
patient suitability.