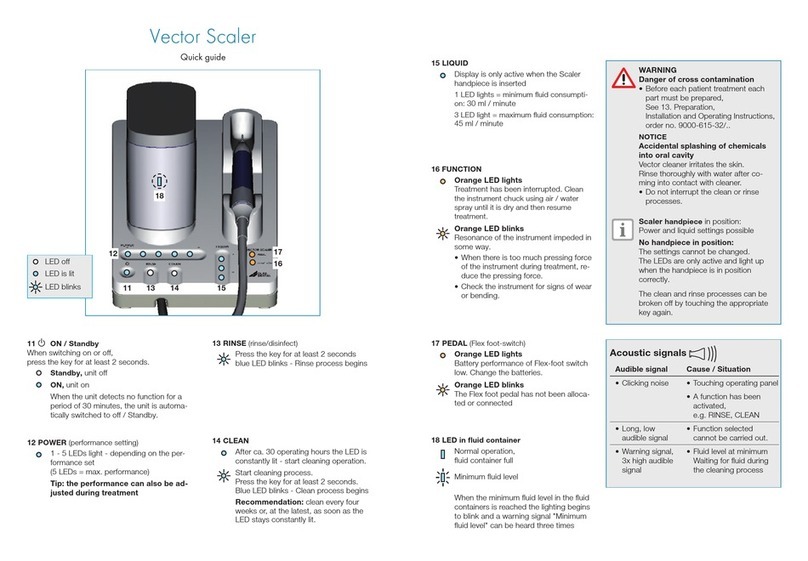
28
IMPORTANT INFORMATION
1. NOTES
1.1 CE - Labeling
CE- Labeling according to Medical Products
Guidelines for Class I Products.
< This product carries the CE - label “CE
0124” according to the Guidelines concerning
Medical Products 93/42/EWG and satisfies all
criteria of Appendix I of these guidelines.
1.2 General Notes
• These Installation and Operating
Instructions form an integral part of the unit.
They must be kept close to the unit and in
readiness whenever required. Precise
observance of these instructions is a pre-
condition for use of the unit for the intended
purpose and for its correct operation.
• Safety for the operator as well as trouble-
free operation of the unit are only ensured if
use is made of original equipment parts.
Moreover, use may only be made of those
accessories that are specified in the
technical documentation or that have been
expressly approved and released by Dürr
Dental for the intended purpose.
Dürr Dental cannot guarantee for the safety or
proper functioning of this unit in the case
where parts or accessories are used which
are not supplied by Dürr Dental.
• There is no guarantee against damage
arising where parts or accessories are used
which are not supplied by Dürr Dental.
• The guarantee is valid for 1 year
commencing from the date of delivery. Any
work performed under guarantee will neither
extend nor renew the guarantee.
• Dürr Dental only regard themselves as being
responsible for the equipment with regard to
safety, reliability and proper functioning if
assembly, resettings, changes or
modifications, extensions and repairs have
been carried out by Dürr Dental or an
agency authorized by Dürr Dental and if the
equipment is used in conformity with the
Installation and Operating Instructions.
• These Installation and Operating
Instructions conform to the relevant version
of the equipment and the underlying safety
standards valid at the time of going to press.
All switches, processes, trade marks,
software programs and appliances named
in this document are registered names.
• Any reprinting of the technical
documentation, in whole or in part, is
subject to prior approval of Dürr Dental
being given in writing.
1.3 Electromagnetic Compatibility
This unit satisfies the standard EN 60601-1-2
“for Electromagnetic Compatibility - Medical
Electrical Appliances”.
1.4 General Safety Information
• Retain the packaging for possible return of
the product to the manufacturers. Ensure
that the packaging is kept out of the reach
of children. Only the original packaging
provides adequate protection during
transport of the unit.
Should return of the product to the
manufacturers be necessary during the
guarantee period, Dürr Dental accepts no
responsibility for damage occurring during
transport where the original packaging was
not used!
• This product is a medical-technical appliance
and may only be operated or used by
someone who has been trained or who has
experience of how to use the appliance
correctly.
• Before every use the operator must check
the functional safety and the condition of
the appliance.
• The operator must be knowledgeable in the
operation of the appliance.
• The product is not designed to be used in
medical treatment areas where there exists
the danger of explosion. Areas where
explosions could occur are those where
flammable anesthetic material, skin cleansers,
oxygen and skin disinfectants are present.
This appliance is not to be used in areas
where the atmosphere could cause fire.