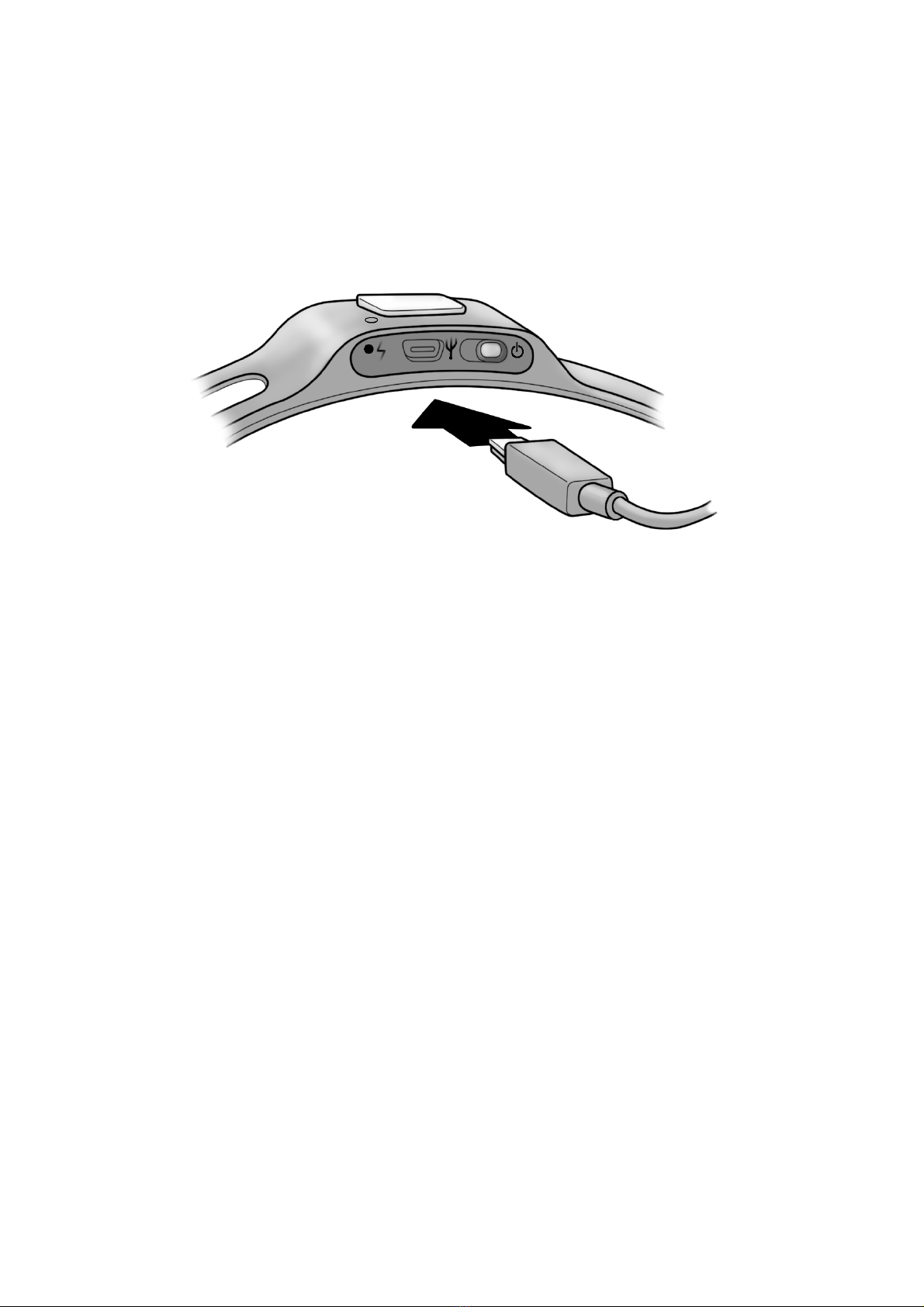
Regulatory Compliance
EMOTIV products are intended to be used for research applications and personal use
only. Our products are not sold as Medical Devices as defined in EU directive 93/42/EEC.
Our products are not designed or intended to be used for diagnosis or treatment of
disease.
The EPOC+ has been tested for EMC and Safety compliance as a consumer product under
the CB scheme. The following table outlines the compliance testing that has been obtained:
FCC ID Number 2ADIH-EPOC02 and IC ID Number: 12769A-EPOC02
EMOTIV has undertaken testing and confirms:
This device complies with the radio equipment directive (2014/53/EU).
This device complies with part 15 of the FCC Rules. Operation is subject to the following
two conditions:
1. This device may not cause harmful interference.
2. This device must accept any interference received, including interference that may cause
undesired operation.
This equipment has been tested and found to comply with the limits for a Class B digital
device, pursuant to part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
equipment generates, uses and radiates radio frequency energy. If it is not installed and
used in accordance with the instructions, it may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and on, the user
is encouraged to try to correct the interference by one or more of the following measures:
- Reorient or relocate the receiving antenna
- Increase the separation between the equipment and receiver
- Connect the equipment into an outlet on a circuit different from that to which the receiver