EMS Physio EMS360 User manual
User Manual
EMS360/460
PRIMO THERASONIC
Model 121/120
1639

OM360/460 Iss 15
2

OM360/460 Iss 15
3
Contents
Page
Contents 3
General information & record of amendments 4
Warranty statement 5
Introduction & indications for use 6
Contraindications 8
Accessories 9
Controls and markings 10
Installation 14
Operating instructions 16
Maintenance 24
Appendix A - Overview of treatment modality 25
Appendix B –Technical specification 27
Appendix C - EMC table 29

OM360/460 Iss 15
4
General Information
This manual provides the necessary information for the installation and
operation of the Primo Therasonic 360 and 460 Units.
These instructions must be studied before putting the unit into operation.
The information contained in this manual is subject to change without notice.
No part of this manual may be photocopied, reproduced or translated into
another language without the prior written consent of EMS Physio Ltd.
Record of Amendments
ISSUE COMMENTS DATE
1 Initial Issue 04/06/09
2 Revised 20/09/10
3 Updated for models 120A/121A 08/04/11
4 Revised declaration of conformity 16/03/12
5 Ref to 21 CFR1050.10 added p.10 25/05/12
6 Updated to show latest images 17/09/12
7 Declaration of conformity revised 26/06/14
8 Updated for colour TFT GUI 09/05/17
9/10/11 Minor edits 15/11/18
12 Corrections 14/12/18
13 D. of C. updated 11/07/19
14 Updated for new NB number 14/04/20
15 Updated for new connectors 07/03/22

OM360/460 Iss 15
5
Warranty
This EMS Physio Ltd., (hereinafter called the Company) product is warranted
against defects in materials and workmanship for a period of two years from
the date of shipment. The Company will at its option, repair or replace
components which prove to be defective during the warranty period, provided
that the repairs or replacements are carried out by the Company or its
approved agents.
The Company will consider itself responsible for the effects on safety,
reliability and performance of the product only if:-
•assembly operations, re-adjustments, modifications or repairs
are carried out by persons authorised by it,
•the product is used in accordance with the instructions for use,
•the electrical installation of the relevant room complies with the
appropriate national requirements.
Should the product be returned to the Company for repair it must be sent
carriage paid.
Consumable items, for example, batteries are excluded from the above
warranty.
It is intended that the Therasonic 360/460 unit is only used by qualified
healthcare professionals such as physiotherapists who have received
training in electrotherapy.

OM360/460 Iss 15
6
Introduction
The Primo Therasonic 360 provides 1MHz ultrasound therapy and the 460
provides both 1MHz and 3MHz ultrasound therapy. The units may be
powered from a (specific) desktop mains to DC PSU or from a suitable
external DC power bank.
Indications for use
Therapeutic ultrasound may be applied to a wide range of conditions with
successful outcomes. These include acute and subacute traumatic and
inflammatory conditions, chronic rheumatoid and arthritic conditions, for pain
relief and for tissue repair during the inflammatory and proliferation stages of
tissue repair.
Precautions
Therapy shall be performed by qualified personnel trained and/or
experienced in the use of this device as outlined in an appropriate training
program.
Electromagnetic interference: This device may cause electromagnetic
interference to electronic devices
The emissions characteristics of this device make it suitable for use in
industrial areas and hospitals (CISPR 11 class A). If it is used in a residential
environment (for which CISPR 11 class B is normally required) this device
might not offer adequate protection to radio-frequency communication
services. The user might need to take mitigation measures, such as
relocating or re-orienting the equipment.
This device is suitable for use in hospital environments except for near active
HF surgical equipment or in the RF shielded room of magnetic resonance
imaging equipment where the intensity of EM disturbances is high.
WARNING: use of this device adjacent to or stacked with other equipment
should be avoided because it could result in improper operation.

OM360/460 Iss 15
7
Cross contamination: Patients with skin infection in the treatment area
should have precautions taken in order to avoid cross-contamination.
The temperature of the ultrasound transducer treatment head may reach 42°
C when operating under maximum operating conditions*.
Maintenance: For continuous and safe operation, regular maintenance and
inspection by EMS authorised technicians is required. For the maintenance
procedures and schedule, refer to the Maintenance section of this manual.
Coupling media: Water-based ultrasound gel should be used as coupling
media between the ultrasound transducers and patient skin.
Cleaning: Proper cleaning of the transducers and main unit is required. For
cleaning instructions, refer to the Maintenance chapter of this manual
Modification of the EMS360/460 is not permitted and may result in a
hazardous situation.
*Transducer temperatures exceeding 42°C will trigger an audible and visible
alarm and will reduce the ultrasound output power until the transducer has
cooled down.

OM360/460 Iss 15
8
Contraindications
Tumours, as ultrasound affects tissue repair and could therefore encourage
growth.
Infections, due to the risk of spreading the infection.
Pregnancy, treatment over the pregnant uterus as ultrasound could affect
rapidly dividing cells.
Radiotherapy, sites that have received radiotherapy treatment during the
last six months.
Thrombosis and impaired circulation.
Areas of impaired sensation.
Haemorrhage, due to the risk of increased bleeding, including recently
controlled bleeding and haematoma.
Haemophilia.
Implanted devices such as cardiac pacemakers should be avoided due
to the possibility of affecting their operation. Also, some plastics used in
replacement surgery may be affected by absorption of ultrasound energy.
Metal implants may lead to reflections, and as a precaution low doses of
ultrasound should be used near these.
Extreme care should be taken when treating areas near the eye because of
the danger of damage to the retina.
Similarly, extreme care should be taken near the ears and reproductive
organs.

OM360/460 Iss 15
9
Accessories supplied as standard
Catalogue
Number
Description
SLA9000
DC power supply 18V 60W
PMA9125
Large dual-frequency transducer
EMS 502C
EMS coupling medium 250ml bottle
Optional Accessories
PMA9126
Large single-frequency (1MHz) transducer
PMA9135
Small dual-frequency transducer
EMS502
EMS coupling medium (8 x 250ml bottles)
EMS502A
EMS coupling medium 1litre bottle
EMS530
Primo shoulder bag
EMS158
Primo trolley
Supplied with each unit is a detachable mains lead suitable for the country
to which it is delivered. Replacement or additional mains leads are shown
below.
EMS Part Number
Description
6-85
UK mains lead
6-112
European mains lead
6-119
North America mains lead
For other countries contact EMS Physio Ltd. (contact details on page 24) or
the agent from whom the unit was purchased.
WARNING: Use of accessories such as transducers, electrodes or mains
cables other than those specified or provided by the manufacturer of this
equipment could result in increased electromagnetic emissions or decreased
electromagnetic immunity of this equipment and result in improper operation.
WARNING: Portable RF communications equipment (including peripherals
such as antenna cables and external antennas) should be used no closer
than 30cm (12 inches) to any part of the EMS360/460 including cables
specified by the manufacturer, otherwise degradation of the performance of
this equipment could result.

OM360/460 Iss 15
10
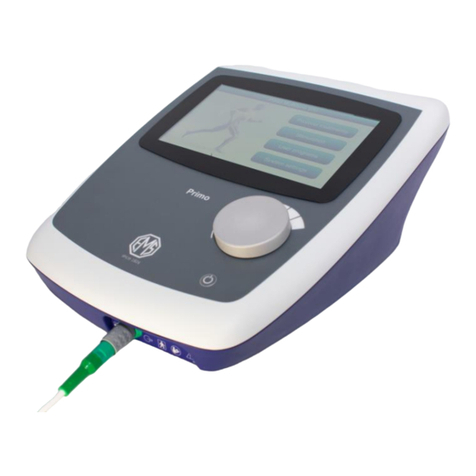
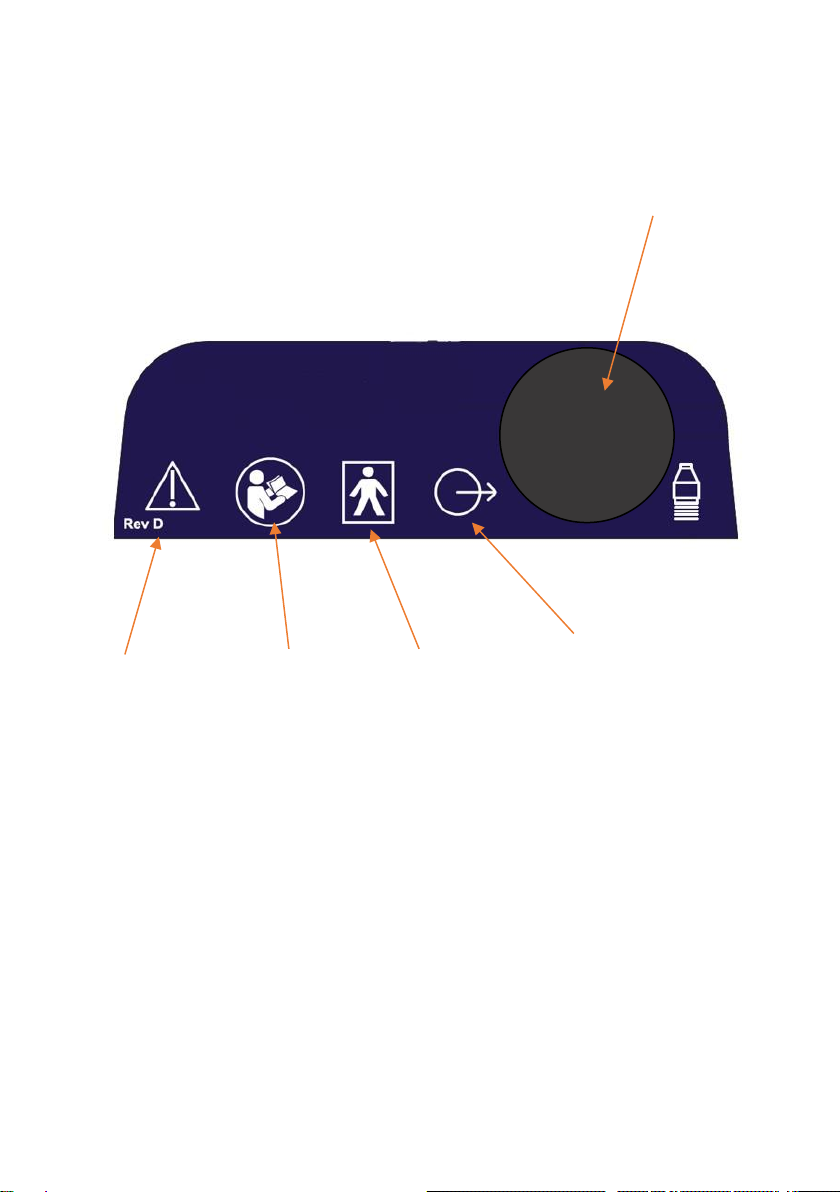
Controls and Markings
Therasonic 360 or 460 Top
TFT display
with
touchscreen
Cradle for ultrasound
transducer
Output
control knob
IEC
symbol
848-01-26
variability
in steps
On/off button
OM360/460 Iss 15
11
Therasonic 360 or 460 Front Label
The output socket is for connection of the ultrasound transducer
Ultrasound
output socket
IEC symbol
878-01-37
Output
IEC symbol
878-02-03 Type
BF equipment
IEC symbol 348
Attention, consult
accompanying
documents
ISO symbol
7010-M002,
consult
instructions for
use
OM360/460 Iss 15
12
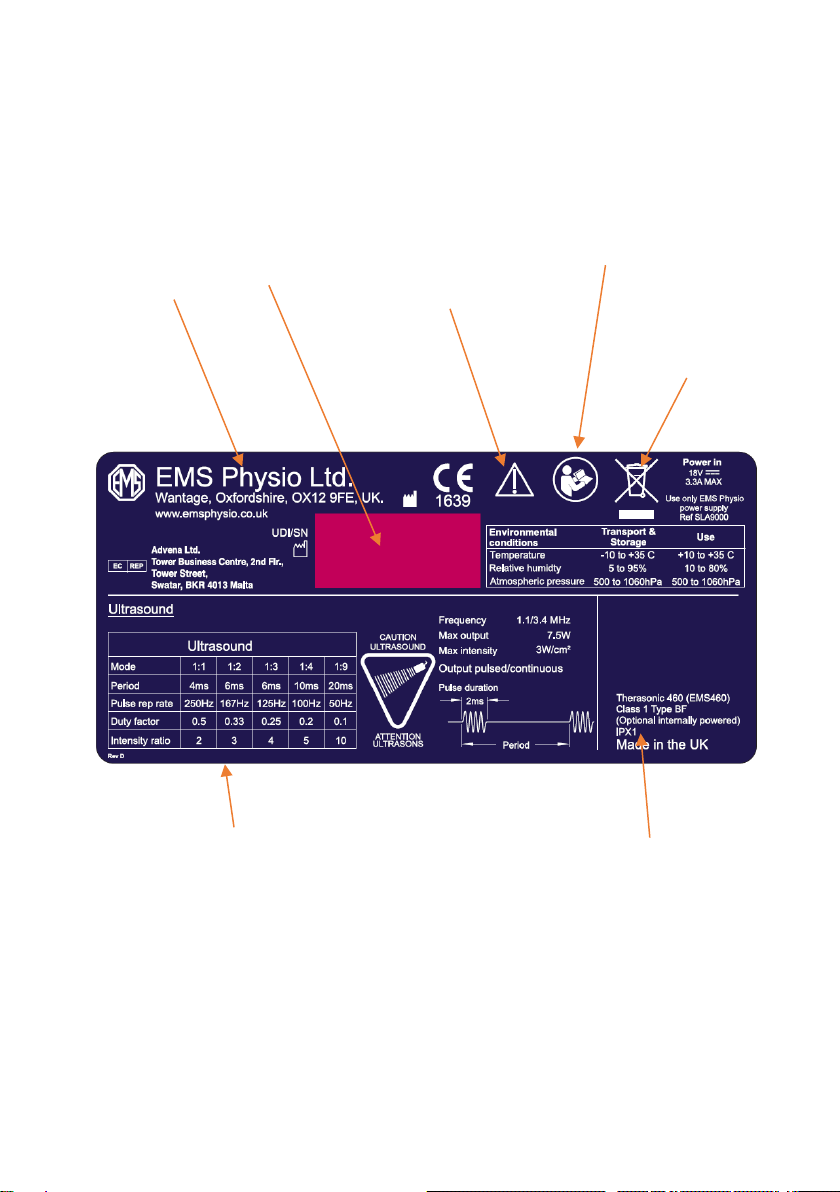
Therasonic 360 or 460 Underside Label
Description
of ultrasound
output
waveform for
each mode
Do not dispose of
as unsorted
waste
(2006/96/EC
WEEE Directive)
Serial number
and date of
manufacture
Name and
address of
manufacturer
IEC symbol 348
Attention, consult
accompanying
documents
ISO symbol 7010-
M002, consult
instructions for
use
Model number
and
classification

OM360/460 Iss 15
13
Treatment light
Large Transducer
Small Transducer
The ultrasound transducers are calibrated independently from the Primo
Therasonic 360/460 and are fully interchangeable.
Treatment light
Active
face
Active
face

OM360/460 Iss 15
14
Installation
Upon receipt, check for any visible damage which may have occurred in
transit. If any signs of damage are found then retain all packing material and
inform the carrier and the Company or its agent from whom the unit was
purchased within two working days.
The Primo Therasonic 360 or 460 operates at 18V and must only be used
with an EMS Physio SLA9000 power supply (as supplied with the unit) which
is connected to a mains supply of 100-240V ac. A power cord appropriately
rated/approved for the country of use must be used.
The SLA9000 power supply must only be connected to a mains supply with
a protective earth conductor. If the integrity of the earth connection is in
doubt, do not connect it to the mains supply (risk of electric shock with type
B applied parts). The unit must not be positioned in such a way that the mains
plug cannot easily be unplugged –the mains plug is the main disconnect
device.
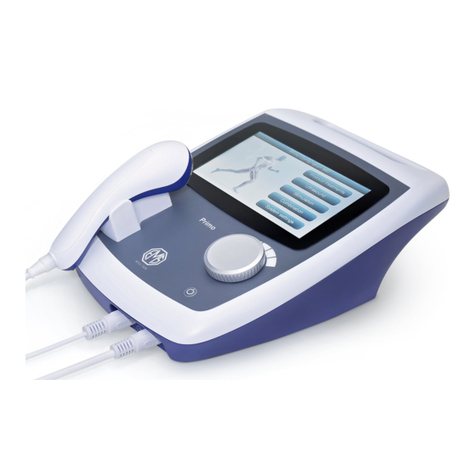
The Primo Therasonic 360 or 460 unit is supplied with a large ultrasound
transducer. An optional small transducer is also available.
Plug the ultrasound transducer into the output socket on the front right of the
unit.
Be careful not to subject the ultrasound transducers to rough handling such
as dropping onto a hard surface as this may impair performance.
Permissible Environmental Conditions Of Use:
Temperature 10 to +35°C
Relative humidity 10 to 80%
Atmospheric pressure 500 to 1060hPa
Permissible Environmental Conditions Of Transport And Storage:
Temperature -10 to +35°C
Relative humidity 5 to 95%
Atmospheric pressure 500 to 1060hPa
Expected Service Life:
7 years

OM360/460 Iss 15
15
Essential Performance
BSEN 60601-1 defines Essential Performance as:
“Performance necessary to achieve freedom from unacceptable risk”
Functions of the EMS360/460, the absence or degradation of which could
result in a hazardous situation are:
•Maximum ultrasound intensity 3W/cm2
•Maximum treatment time 30 minutes
Loss or degradation of these functions due to EM disturbances (eg.
electrostatic discharges or mains voltage dips) may cause temporary loss of
output but this is not considered to be hazardous.

OM360/460 Iss 15
16
Operating Instructions
Power On Sequence and General Information
After the Primo Therasonic 360/460 is turned on a splash screen appears
showing the EMS company logo along with the model name, its serial
number and the installed software version.
After a few seconds the unit will give a short beep and display the Home
screen.
OM360/460 Iss 15
17
Standard User Controls
Throughout the operation of the Therasonic 360/460 the various modes and
parameter settings are all accessed and changed by touching the relevant
buttons displayed on the touchscreen.
The rotary control is used to increase and decrease the ultrasound intensity
when the display is showing the ultrasound screen. It can also be used to
safely stop a treatment by turning it all the way anticlockwise.
On most display screens touching the back arrow icon in the top left corner
will return the user to the last screen displayed, and touching the house icon
in the top right corner will return the display to the main Home screen.
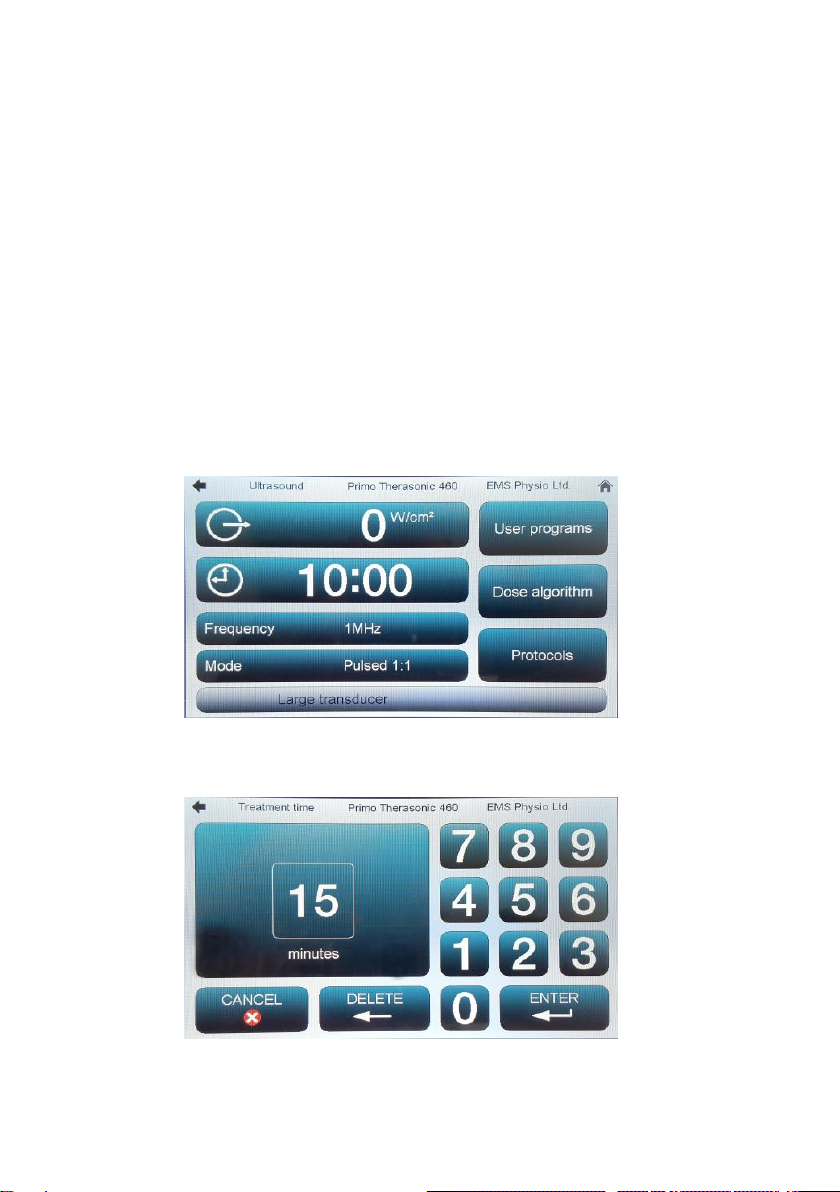
Ultrasound Set Up
From the Home screen, touch the button marked ‘Ultrasound’. The
Ultrasound set-up screen will appear.
Touch the screen on the digits of the time display to increment some
treatment time (maximum 30 minutes). Alternatively, touch the clock symbol
to bring up the following screen:-
Type in the desired time and touch ENTER to return to the main screen.

OM360/460 Iss 15
18
Select the desired ultrasound Frequency* and Mode (pulsed 1:9, 1:4, 1:3,
1:2, 1:1 or continuous) by touching the relevant field on the screen.
*The Frequency button on the 360 single-frequency unit will be set to 1MHz
and greyed-out.
Ultrasound Treatment
It is recommended that before commencing treatment, the stainless steel
front of the transducer is disinfected using a 70% v/v aqueous solution of
isopropyl alcohol. Sterile alcohol wipes are suitable for this purpose.
Apply sufficient coupling medium to the area to be treated, EMS Physio
Therasonic coupling medium is recommended.
Apply the active face of the transducer to the treatment site via the coupling
medium.
Turn the rotary control clockwise to start treatment. The output intensity will
increase in 0.1 W/cm2steps. The treatment indicator on the transducer will
light, ‘TREATMENT’ will flash at the bottom of the screen and the treatment
time will begin to count down.
If the transducer is not properly connected to the output socket or the
treatment time is zero then the unit will give a two-tone beep and the output
will not be energised.
Move the transducer over the treatment site in small circular paths whilst
setting the output intensity to the required level using the rotary control.

OM360/460 Iss 15
19
Always keep the face of the transducer in contact with the treatment area
and always keep the transducer moving to avoid any standing waves.
If the transducer face is lifted from the treatment site or if for any reason there
is insufficient contact between the transducer and the treatment site for more
than two seconds, the power applied to the transducer will be reduced to a
low level. The treatment light on the transducer will start flashing, the
treatment time will cease to count down and the status bar will display
‘CONTACT’, indicating that the required output cannot be delivered. An
audible alarm will sound if this option has been selected in the Setup menu.
When good contact is restored, the treatment indicator on the transducer will
relight, the status bar will display ‘TREATMENT’and the timer will continue
to count down.
If the output intensity is returned to zero using the rotary control, before the
treatment time has elapsed, the display will show the treatment time
remaining. When the intensity is increased again the treatment will continue.
When the treatment time reaches ‘00:00’, treatment is terminated. The
intensity and power displays will go to zero, ultrasonic power from the
transducer will be turned off, the treatment indicator will turn off and the unit
will give a two second beep. Remove the transducer from the treatment site,
wipe off any coupling medium and return the transducer to its cradle on the
front of the unit.
Remove the remaining coupling medium from the treatment site.
The transducers are also suitable for treatment using a water bath. This is
especially useful when treating areas which are not uniform such as feet or
hands. When using a water bath it is advisable to use degassed water (water
that has been boiled to remove any air and then allowed to cool). After the
part of the body has been immersed in the water, remove any air bubbles
that may have accumulated on the skin. Set up the treatment parameters
and then immerse the transducer in the water before turning the output on.
Hold the transducer with its face approximately 1 cm away from the treatment
site and using the rotary control set the required intensity remembering to
keep the transducer moving in small circular paths to prevent standing
waves. At the end of the treatment the intensity and power displays will read
zero, and the ultrasound power will turn off. Remove the transducer from the
water and dry both it and the area treated.

OM360/460 Iss 15
20
Protocol Treatments
Touching the ‘Protocols’ button in the ultrasound set-up screen will open a
screen with a scrollable list of clinical conditions and front/back human body
image. Touching different parts of the body will select a list of conditions
specific to that body area.
Touching the highlighted condition in the list will open a user screen with the
treatment parameters set for treating that condition.
Most parameters in a protocol treatment screen will be ‘greyed-out’ and not
adjustable by the user.
This manual suits for next models
3
Table of contents
Other EMS Physio Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual