EMS Physio EMS860 User manual
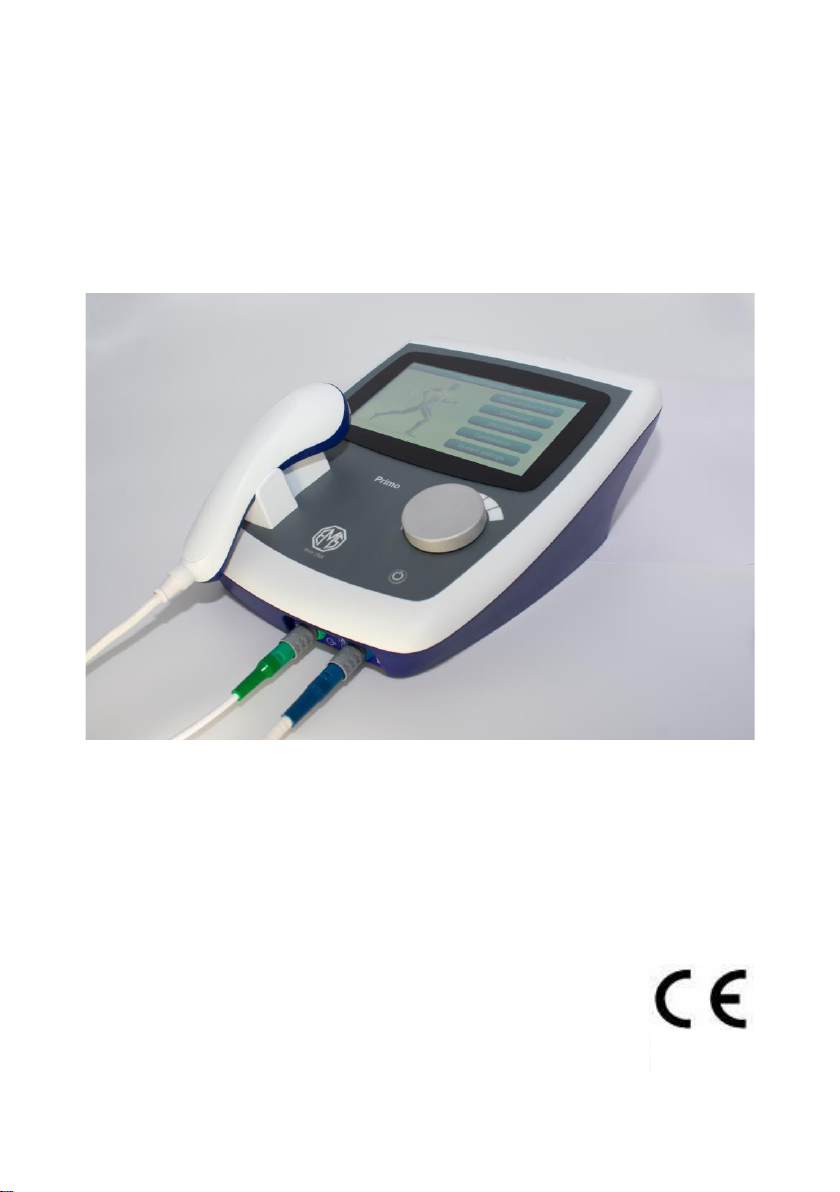
User Manual
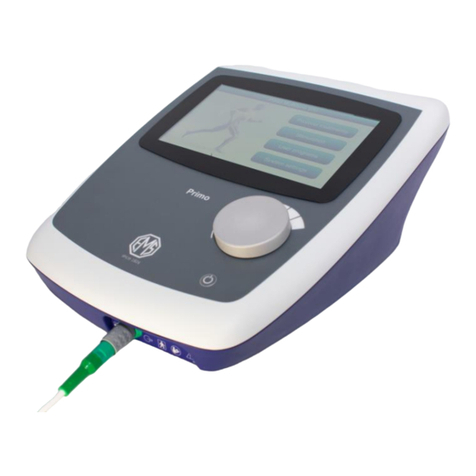
EMS860
PRIMO COMBINATION
Model 125
1639

OM860EN Iss 13
2

OM860EN Iss 13
3
Contents
Page
Contents 3
General information & record of amendments 4
Warranty statement 5
Introduction & indications for use 6
Contraindications 9
Accessories 11
Controls and markings 13
Installation 18
Operating instructions 20
Ultrasound 23
Stimulation 28
Combination 50
Electrodes 52
Maintenance 53
Appendix A - Overview of treatment modalities 54
Appendix B –Technical specification 60
Appendix C - EMC table 73
Appendix D –Electrotherapy chart 74

OM860EN Iss 13
4
General Information
This manual provides the necessary information for the installation and
operation of the Primo Combination 860 unit.
These instructions must be studied before putting the unit into operation.
The information contained in this manual is subject to change without notice.
No part of this manual may be photocopied, reproduced or translated into
another language without the prior written consent of EMS Physio Ltd.
Record of Amendments
ISSUE COMMENTS DATE
1 Initial issue 31/03/11
2 Errata corrected 07/04/11
3 Combination therapy instructions 30/06/11
corrected.
4 Indications for use added 21/06/12
5 Updated to show latest images 08/10/12
6 Declaration of conformity revised 26/06/14
7 Technical revisions 15/06/15
8 Updated for colour TFT GUI 08/02/17
9 Minor edits 05/07/17
10 Small transducer constraint in Combi. 15/11/18
11 Corrections 14/12/18
12 Updated for new NB number 27/03/20
13 Updated for independent channel stim. 30/09/21

OM860EN Iss 13
5
Warranty
This EMS Physio Ltd., (hereinafter called the Company) product is warranted
against defects in materials and workmanship for a period of two years from
the date of shipment. The Company will at its option, repair or replace
components which prove to be defective during the warranty period, provided
that the repairs or replacements are carried out by the Company or its
approved agents.
The Company will consider itself responsible for the effects on safety,
reliability and performance of the product only if:-
assembly operations, re-adjustments, modifications or repairs are carried
out by persons authorised by it,
the product is used in accordance with the instructions for use,
the electrical installation of the relevant room complies with the
appropriate national requirements.
Should the product be returned to the Company for repair it must be sent
carriage paid.
Consumable items, for example, electrodes, electrode covers and batteries
are excluded from the above warranty.
It is intended that the Combination 860 unit is only used by qualified
healthcare professionals such as physiotherapists who have received
training in electrotherapy.

OM860EN Iss 13
6
Introduction
The Primo Combination 860 provides 1 and 3 MHz ultrasound and two
independent electrical stimulation channels each with a complete range of
low and medium frequency waveforms for electrotherapy. Both modalities
may be used individually or in combination. The unit may be powered from a
(specific) desktop mains to DC PSU or from a suitable external DC power
bank.
Indications for use
Therapeutic ultrasound may be applied to a wide range of conditions with
successful outcomes. These include acute and subacute traumatic and
inflammatory conditions such as chronic rheumatoid and arthritic conditions,
for pain relief and for tissue repair during the inflammatory and proliferation
stages of tissue repair.
The Primo Combination 860 unit also provides 4 pole and 2 pole interferential
therapy as well as a wide range of other electrical stimulation waveforms.
Voltage and current waveforms may be used to provide Neuro Muscular
Electrical Stimulation (NMES) and relief from musculoskeletal pain. NMES
may be used for muscle strengthening and rehabilitation in otherwise healthy
subjects recovering from surgery, for muscle strengthening for critically or
chronically ill patients or to (re)train weak or ineffective muscles.
Pain relief may be appropriate post-surgery during rehabilitation, or for relief
from chronic conditions such as osteoarthritis.
The various output waveforms available from the units are suitable for either
NMES and/or pain relief as shown in the chart in Appendix D on page 74.
Combination therapy involves the simultaneous application of ultrasound
with an electrical stimulation therapy.
By combining ultrasound with interferential therapy, the advantages and
effects of each treatment modality can be realised - but lower intensities are
used to achieve the effect. The accommodation effects that normally
accompany interferential therapy are reduced (or even eliminated). The main
advantages of such a combination are in localising lesions (especially
chronic), in ensuring accurate localisation of ultrasound treatment to provide
increased accuracy/effectiveness in treating deeper lesions, and in treating
trigger points.

OM860EN Iss 13
7
Precautions
Therapy shall be performed by qualified personnel trained and/or
experienced in the use of this device as outlined in an appropriate training
program.
Electromagnetic interference: This device may cause electromagnetic
interference to electronic devices
The emissions characteristics of this device make it suitable for use in
industrial areas and hospitals (CISPR 11 class A). If it is used in a residential
environment (for which CISPR 11 class B is normally required) this device
might not offer adequate protection to radio-frequency communication
services. The user might need to take mitigation measures, such as
relocating or re-orienting the equipment.
This device is suitable for use in hospital environments except for near active
HF surgical equipment or in the RF shielded room of magnetic resonance
imaging equipment where the intensity of EM disturbances is high.
WARNING: use of this device adjacent to or stacked with other equipment
should be avoided because it could result in improper operation.
Cross contamination: Patients with skin infection in the treatment area
should have precautions taken in order to avoid cross-contamination.
Consideration must be given to the current densities for any electrode used
with the Combination 860 unit. Current densities greater than 2 mA rms/cm2
are not recommended because of the risk of burning. All the standard EMS
Physio conductive rubber electrodes may be used up to the maximum output
of the unit without exceeding this figure. When using other electrodes, the
maximum safe output current should be assessed before use. First estimate
the effective contact area of the electrode in square cm, and then apply the
following formula: -
rms output current (mA) = Area of electrode (cm2) x 2
The ratio of the rms to the peak current for the different operating modes is
given in the technical specification section of this manual.

OM860EN Iss 13
8
The output indication on the display screen shows the peak output voltage
or the peak output current in mA depending upon the selected mode of
operation.
When using direct current, extreme care must be taken to ensure the
patient's safety from electrochemical burning. In particular care must be
taken to avoid uneven pressure on the electrodes causing high local current
density.
Electrodes must not be applied where there are cuts or abrasions.
The temperature of the ultrasound transducer treatment head may reach 42°
C when operating under maximum operating conditions*.
Maintenance: For continuous and safe operation, regular maintenance and
inspection by EMS authorised technicians is required. For the maintenance
procedures and schedule, refer to the Maintenance section of this manual.
Coupling media: Water-based ultrasound gel should be used as coupling
media between the ultrasound transducers and patient skin.
Cleaning: Proper cleaning of the transducers, electrodes and main unit is
required. For cleaning instructions, refer to the Maintenance chapter of this
manual
Modification of the EMS860 is not permitted and may result in a hazardous
situation.
*If the transducer temperature exceeds 42° C then a detector in the
transducer sounds and displays an alarm message on the display screen
and ultrasound power is reduced to a low level until the transducer has
cooled down sufficiently.

OM860EN Iss 13
9
Contraindications - Ultrasound
Tumours, as ultrasound affects tissue repair and could therefore encourage
growth.
Infections, due to the risk of spreading the infection.
Pregnancy, treatment over the pregnant uterus as ultrasound could
affect rapidly dividing cells.
Radiotherapy, sites that have received radiotherapy treatment during the
previous six months.
Thrombosis and impaired circulation.
Areas of impaired sensation.
Haemorrhage, due to the risk of increased bleeding, including recently
controlled bleeding and haematoma.
Haemophilia.
Implanted devices such as cardiac pacemakers should be avoided due
to the possibility of affecting their operation. Some plastics used in
replacement surgery may be affected by absorption of ultrasound energy.
Metal implants may lead to reflections, and as a precaution low doses of
ultrasound should be used near these.
Extreme care should be taken when treating areas near the eye because of
the danger of damage to the retina.
Similarly, extreme care should be taken near the ears and reproductive
organs.

OM860EN Iss 13
10
Contraindications –Electrotherapy
Acute Sepsis, due to the risk of spreading infection.
Tumours, due to the risk of increased growth or metastatic activity.
Pregnancy, do not treat the lower abdomen, back or pelvis.
Menstruation, do not treat lower back or abdomen due to risk of increased
bleeding or pain.
Cardiac conditions, do not treat the chest area, across the heart or near
the cervical ganglion –may cause cardiac fibrillation.
Cardiac pacemakers, especially demand type, or any other implanted
electronic device, unless specialist medical opinion has first been obtained.
Febrile conditions.
Large open wounds in treatment area.
Dermatological conditions in treatment area.
Thrombosis.
Hypersensitivity or fear of electrical treatments.
Any patient who cannot understand the nature of the treatment, for
example, young children, very old or senile patients who cannot report back
adequately or understand the potential dangers. This may apply equally to
persons who do not speak the same language as the therapist.
Severe hypotension/hypertension, do not treat in the region of the lower
cervical spine.
If in doubt the patient's physician should be consulted.
Electrodes should never be placed so that the applied current goes
across or through the head, eye, front of the neck (especially the
carotid sinus), upper back or chest.
Electrodes must never cover the mouth.

OM860EN Iss 13
11
Accessories supplied as standard
Optional Accessories
Catalogue
number
Description
SLA9000
DC power supply 18V 60W
PMA9125
Large dual-frequency transducer
EMS502C
EMS Physio coupling medium (250ml bottle)
PMA3055
Patient lead (4 way –yellow and blue connecting
cables included)
NC3053A
4 medium sponge electrode covers (for
NC3053B)
NC3053B
4 medium (100 x 70 mm) conductive rubber
electrodes
DU2
2 stretch bandages 1200 x 75 mm
EMS530
Primo shoulder bag
EMS158
Primo trolley
PMA9135
Small dual-frequency transducer
EMS502
EMS Physio coupling medium (8 x 250ml bottles)
EMS502A
EMS Physio coupling medium 1litre bottle
NC3052A
4 small sponge electrode covers (for NC3052B)
NC3052B
4 small (70 x 50 mm) conductive rubber
electrodes
NC3054A
4 large sponge electrode covers (for NC3054B)
NC3054B
4 large (130 x 100 mm) conductive rubber
electrodes
NC3041
Electrode handle (for circular pad & ball
electrodes)
NC3042A
Connecting cable for electrode handle
NC3046
Circular pad electrode 12 mm diameter
NC3048
Circular pad electrode 37 mm diameter
NC311A
Ball electrode for muscle testing
DU1
Stretch bandage 600 x 75 mm
DU4
Stretch bandage 600 x 50 mm

OM860EN Iss 13
12
A range of single-patient self-adhesive electrodes is available
Catalogue
Number
Description
RB410
33 x 54 mm (pack of 4)
RB430
50 x 50 mm (pack of 4)
RB440
80 x 100 mm (pack of 2)
RB450
25 mm diameter round (pack of 4)
Supplied with each unit is a detachable mains lead suitable for the country
to which it is delivered. Replacement or additional mains leads are shown
below.
EMS Part Number
Description
6-85
UK mains lead
6-112
European mains lead
6-119
North America mains lead
For other countries contact EMS Physio Ltd. (contact details on page 53) or
the agent from whom the unit was purchased.
WARNING: Use of accessories such as transducers, electrodes or mains
cables other than those specified or provided by the manufacturer of this
equipment could result in increased electromagnetic emissions or decreased
electromagnetic immunity of this equipment and result in improper operation.
WARNING: Portable RF communications equipment (including peripherals
such as antenna cables and external antennas) should be used no closer
than 30cm (12 inches) to any part of the Combination 860 including cables
specified by the manufacturer, otherwise degradation of the performance of
this equipment could result.

OM860EN Iss 13
13
Controls and Markings
Primo Combination 860 Top
IEC symbol
848-01-26
variability
in steps
On/Off button
Cradle for
ultrasound
transducer
Output control
knob
TFT display
with
touchscreen
OM860EN Iss 13
14
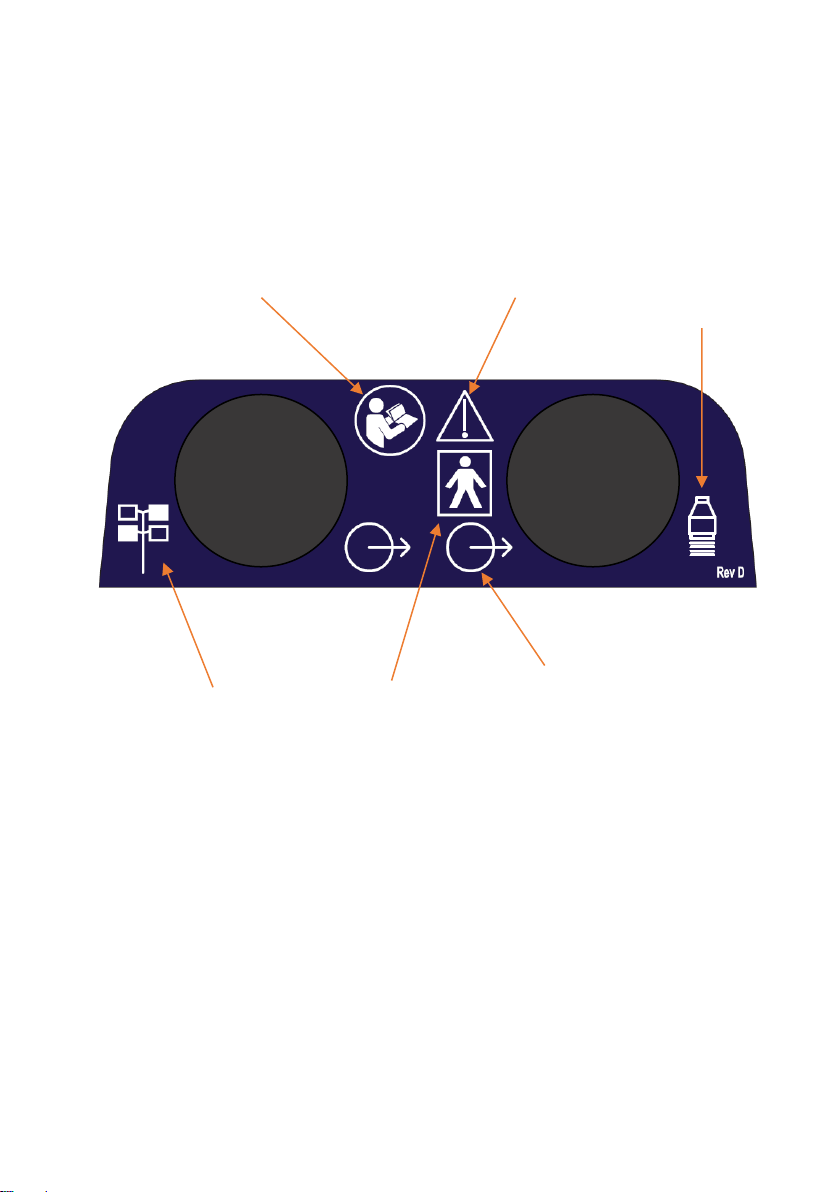
Primo Combination 860 Front Label
Ultrasound
output
IEC symbol
878-01-37
Output
IEC symbol 348
Attention, consult
accompanying
documents
ISO symbol 7010-M002
Consult instructions for use
IEC symbol
878-02-03 Type
BF equipment
Stimulator
output
OM860EN Iss 13
15
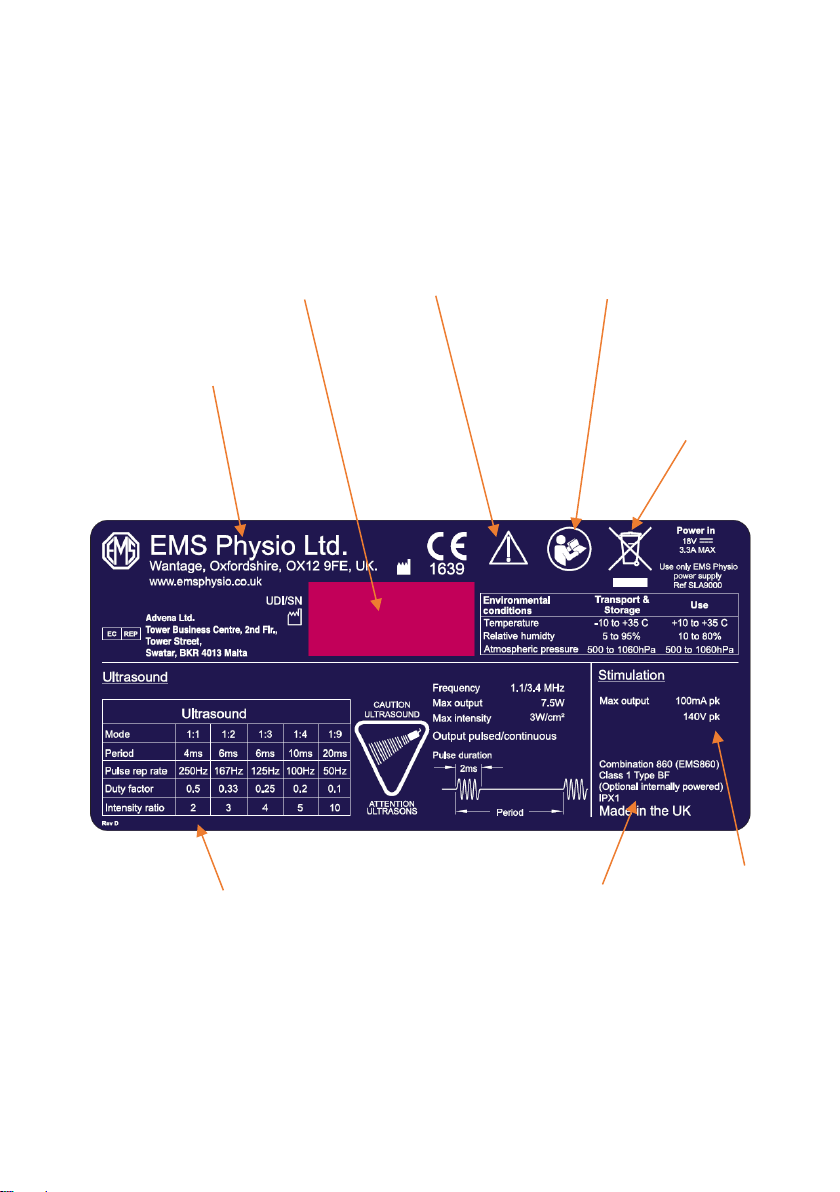
Primo Combination 860 Underside Label
Description of
ultrasound output
waveform for each
mode
Do not dispose
of as unsorted
waste
(2006/96/EC
WEEE Directive)
Serial
number and
date of
manufacture
Stimulation
output levels
Name and
address of
manufacturer
IEC symbol 348
Attention, consult
accompanying
documents
ISO symbol 7010-M002,
consult instructions for
use
Model number
and
classification

OM860EN Iss 13
16
Large Transducer
Small Transducer
The ultrasound transducers are calibrated independently from the Primo
Combination 860 and are fully interchangeable.
Treatment light
Active
face
Treatment light
Active
face

OM860EN Iss 13
17
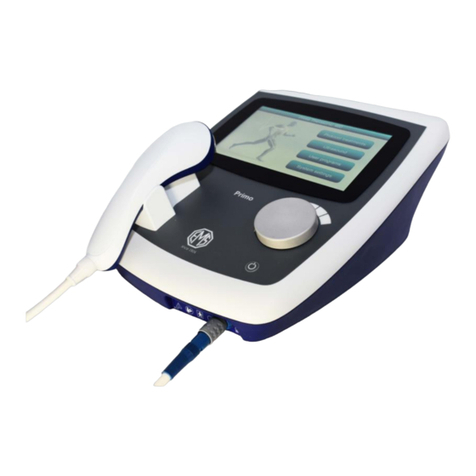
Patient Lead (PMA3055)
Electrode connecting
cables

OM860EN Iss 13
18
Installation
Upon receipt, check for any visible damage which may have occurred in
transit. If any signs of damage are found then retain all packing material and
inform, within two working days, the carrier and the Company or its agent
from whom the unit was purchased.
The Primo Combination 860 operates at 18Vdc and if mains driven must only
be used with an EMS Physio SLA9000 power supply (as supplied with the
unit) which is connected to a mains supply of 100-240V ac. A power cord
appropriately rated/approved for the country of use must be used.
The SLA9000 power supply must only be connected to a mains supply with
a protective earth conductor. If the integrity of the earth connection is in
doubt, do not connect it to the mains supply (risk of electric shock with type
B applied parts). The unit must not be positioned in such a way that the mains
plug cannot easily be unplugged as the mains plug is the main disconnect
device.
The Primo Combination 860 unit is supplied with a large ultrasound
transducer and four medium-sized electrotherapy electrodes with their
associated patient lead. An optional small transducer is also available.
Plug the ultrasound transducer into the output socket on the front right of the
unit and the patient lead into the one to its left and connect the electrodes to
the yellow and blue cables.
Be careful not to subject the ultrasound transducers to rough handling such
as dropping onto a hard surface as this may impair performance.
Operation of the unit in close proximity (less than 1 metre) to shortwave
therapy equipment or radio-frequency mobile communication equipment
could result in the stimulation output being affected.
Permissible Environmental Conditions Of Use:
Temperature +10 to +35°C
Relative humidity 10 to 80%
Atmospheric pressure 500 to 1060hPa

OM860EN Iss 13
19
Permissible Environmental Conditions For Transport And Storage:
Temperature -10 to +35°C
Relative humidity 5 to 95%
Atmospheric pressure 500 to 1060hPa
Expected Service Life:
7 years
Essential Performance
BS EN 60601-1 defines Essential Performance as:
“Performance necessary to achieve freedom from unacceptable risk”
Functions of the Combination 860, the absence or degradation of which
could result in a hazardous situation are:
Maximum ultrasound intensity 3W/cm2
Maximum stimulation output 100mA CC or 140V CV
Maximum treatment time 30 minutes
Loss or degradation of these functions due to EM disturbances (eg.
electrostatic discharges or mains voltage dips) may cause temporary loss of
output but this is not considered to be hazardous.

OM860EN Iss 13
20
Operating instructions
Power On Sequence and General Information
After the Primo Combination 860 is turned on a splash screen appears
showing the EMS company logo along with the model name, its serial
number and the installed software version.
After a few seconds the unit will give a short beep and display the ‘Home’
screen.
This manual suits for next models
1
Table of contents
Other EMS Physio Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Bioness
Bioness NESS L300 Plus user guide

Mentalab
Mentalab EXPLORE quick start guide

BRAINTRONICS
BRAINTRONICS BRAINBOX EEG-1142 user manual

chinesport
chinesport 06855 User and maintenance manual

NeuroMetrix
NeuroMetrix NC-stat DPNCheck reference guide

Luxfer
Luxfer EasyPulse 1901-765 Series Instructions for use