FagronLab™ PRO – User Manual | 10
FagronLab™ assortment
FagronLab™ stirrer
Assignment of the FagronLab™ stirrer
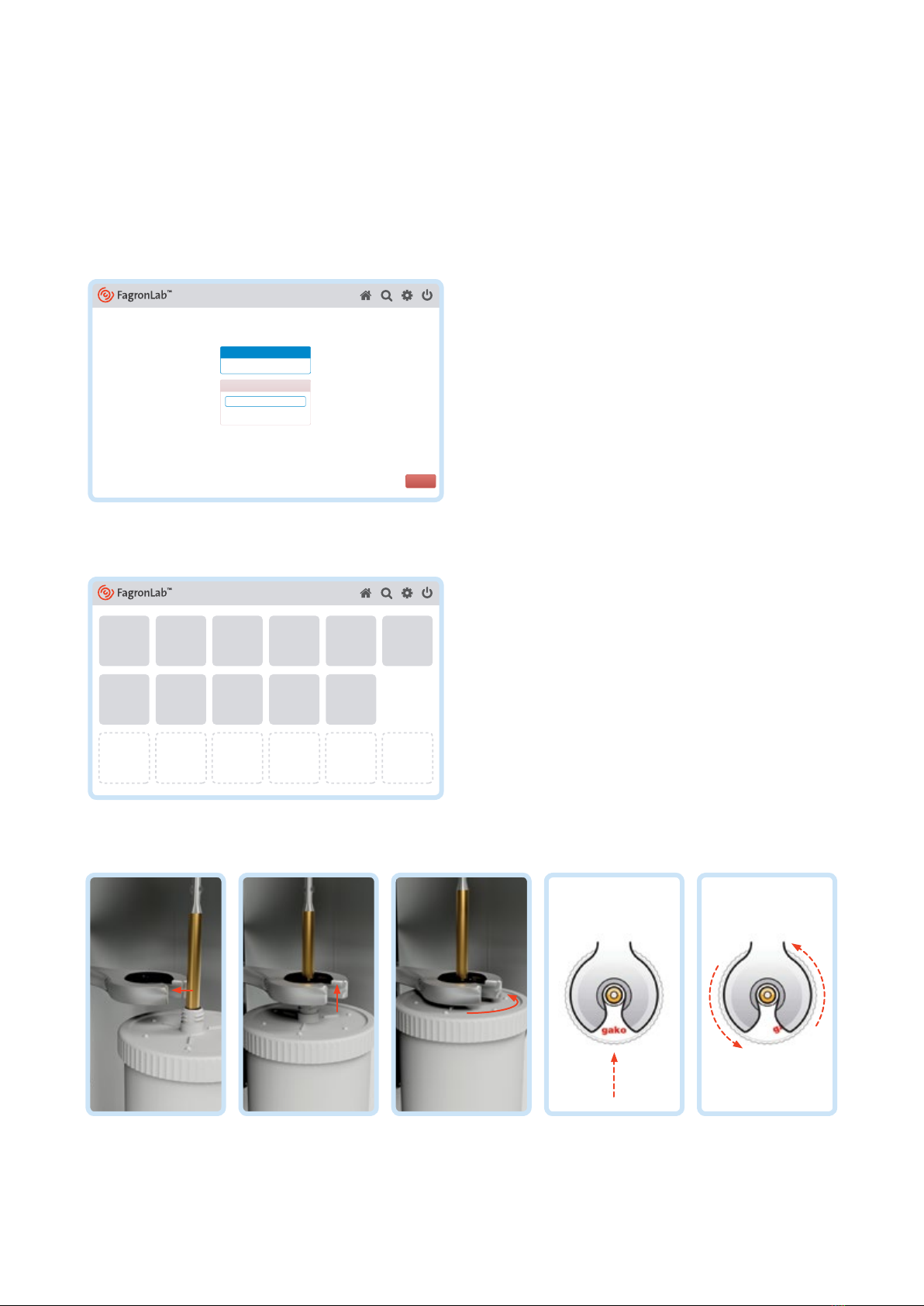
Flowing recess of the FagronLab™ SMB
Warming
Cleaning the FagronLab™ stirrer
As well as the FagronLab™ devices, the FagronLab™ assortment underlies further and new developments. All FagronLab™
accessories are compatible with all current and former available FagronLab™ mixing devices.
The FagronLab™ stirrer are the FagronLab™ Standard Mixing Blade (SMB) and the FagronLab™ Disposable Blade (Disp. Blade).
The stirrers are steadily guided up and down inside the jar. Their unique design results in a tight contact between the mixing
blade and the inside wall of the jar, which serves, primarily, for distributing the substances during the mixing process.
The lubrication eect of the ointment and the foundation generally protects the jars and the stirrer against abrasion.
Discolorations of the mixing blade are mostly irreversible and therefore harmless. All SMB and Disp. Blade shafts are
dishwasher safe.
The SMB and mixing shaft of the disposable blades are coated with titanium nitride, which makes them more resistant
towards chemical and physical influences.
Ensure to use the correct stirrer for the corresponding jar. Selecting the wrong shaft may cause failure messages with the
automated devices. Also ensure that the right shaft is used when working with the Disp. Blade. Both available shafts are
marked for use with sizes 15-100 ml or 200 ml in the FagronLab™ jar. They have to be combined with the correct Disp. Blade.
While the same Disp. Blade is used for the 100 ml and 200 ml jar sizes, it still needs a dierent shaft for each. See also the
operation instructions that come with the disposable blade shafts.
The flow-adapted shape of the SMB generally cleans itself during the rotating penetration of the ointment. Depending
on ointment’s ingredients’, compatibility of weighted formulation and also if the jar is considerably under filled (e. g. large
volumes of powder), unmixed ingredients may adhere to the SMB in recesses of flow. These remnants should be transferred
into the jar using a spatula when about half of the mixing time is complete.
The air should be diminished again, by pushing the bottom of the jar up, following this process. When using the Disp. Blade,
however there are no recesses of flow and no remedial work is generally required.
The warmth that develops from the friction between the stirrer and the inside wall of the jar is generally desired. Decreased
viscosity increases the wettability of powders and accelerates the penetration of potential powder pockets. Even the
emulsifying ability of fats and oils benefits by warming.
A temperature of 54°C/129°F was the maximum taken after 6 minutes of mixing a highly pasty preparation made of vaseline
and zinc oxide aa at full speed. This temperature increase is generally safe for the substances used in the pharmaceutical
field. Ointments of low viscosity only heat slightly. Volatile substances such as ethereal oils or alcohol do not evaporate from
the closed mixing unit.
The stirrer is normally cleaned with a paper towel and, if necessary, held under hot water faucet and then dried with a paper
towel. The stirrers can also be cleaned in the dishwasher.
The FagronLab™ devices as well as the FagronLab™ line of products should never be treated with sharp-edged objects or
abrasive cleaning agents.
FagronLab™ PRO