Fesia Grasp User manual
Instructions for Use
Fesia Grasp


Instructions for Use
Guide
Fesia Grasp
Rev.: 3.00
Date: 02/2020
English

MANUFACTURER:
Fesia Technology S.L.
Pº Mikeletegi, 1
20009 Donostia / San Sebastián
Spain
MAIL: support@fesiatechnology.com
WEB: www.fesiatechnology.com
SUPPORT: https://fesiatechnology.freshdesk.com/support/home
COMERCIALIZED BY:
Fesia Technology S.L.
Pº Mikeletegi, 1
20009 Donostia / San Sebastián
Spain
0051

INDEX
1General information........................................................................... 1
1.1 Indications of use .................................................................................................................................................1
1.2 Contraindications .................................................................................................................................................1
1.3 Warnings...............................................................................................................................................................1
1.4 Precautions...........................................................................................................................................................1
1.5 Adverse reactions.................................................................................................................................................2
2Symbols and definitions ..................................................................... 3
3Fesia Grasp system description .......................................................... 5
3.1 General description..............................................................................................................................................5
3.2 System components.............................................................................................................................................6
4Fesia Grasp operating instructions ..................................................... 7
4.1 Placement.............................................................................................................................................................7
4.2 Turning On/Off .....................................................................................................................................................8
4.3 Luminous/acoustic indicators..............................................................................................................................9
4.3.1 Stimulator.........................................................................................................................................................9
4.4 Battery charge ......................................................................................................................................................9
4.5 Electrode replacement ........................................................................................................................................9
4.6 Skin care..............................................................................................................................................................10
5Software application ........................................................................ 11
5.1 General description............................................................................................................................................11
5.2 Installation ..........................................................................................................................................................11
5.3 Log in...................................................................................................................................................................11
5.4 Logging in............................................................................................................................................................12
5.5 Bluetooth connection to the device..................................................................................................................13
5.5.1 Connecting to a device..................................................................................................................................13
5.6 Patient management .........................................................................................................................................13
5.6.1 Creating a Patient Profile ..............................................................................................................................13
5.6.2 Selecting a patient .........................................................................................................................................14
5.7 Fesia Grasp protocols.........................................................................................................................................15
5.8 Training protocol................................................................................................................................................15
5.9 Grasp protocol....................................................................................................................................................17
5.9.1 Configuration .................................................................................................................................................17
5.9.2 Grasp ..............................................................................................................................................................19
5.10 Sessions...............................................................................................................................................................20
5.11 Settings ...............................................................................................................................................................22
5.12 Bluetooth connection/disconnection ...............................................................................................................22

5.13 Daily use..............................................................................................................................................................23
6Maintenance and cleaning ............................................................... 25
a) Transport.................................................................................................................................................................25
b) Disposal of equipment and accessories.................................................................................................................25
7Troubleshooting............................................................................... 26
8Technical information ...................................................................... 27
a. Stimulator specifications ........................................................................................................................................27
b. Electrode specifications..........................................................................................................................................28
c. Charger specifications ............................................................................................................................................28
d. EMI Tables...............................................................................................................................................................28
8.1.1 Guidance and manufacturer’s declaration– electromagnetic emissions...................................................29
8.1.2 Guidance and manufacturer’s declaration – electromagnetic immunity ..................................................30
8.1.3 Recommended separation distance between portable and mobile rf communications equipment and the
Fesia Grasp device.......................................................................................................................................................31
9Contact information......................................................................... 32
Instructions for Use: Fesia Grasp
1
1GENERAL INFORMATION
1.1 Indications of use
Fesia Grasp is an electrical stimulation device designed to provide flexion and extension of the wrist and fingers in people
with motor dysfunction of the hand after an injury or disease of the central nervous system. During grasping, Fesia Grasp
electrically stimulates the appropriate muscles of the affected forearm to provide flexion and extension of the wrist and
fingers to improve the grip of the individual. Clinical benefits of functional electrical stimulation may also include
reinforcement of motor relearning, muscle strengthening, prevention/delay of muscle atrophy, increased local blood
flow and maintenance/increase of range of motion.
1.2 Contraindications
•People with cardiac pacemakers or other electrical or metallic implants, unless recommended
by a physician.
•People with severe epilepsy or a recent history of frequent seizures.
•Tumors or cancerous lesions in the area where electrical stimulation is applied.
1.3 Warnings
•Do not use this device to control machines, drive or perform any activity where an involuntary
movement could pose a risk of injury.
•Do not place the electrode on the head, eyes, mouth, throat (carotid sinus), chest or back.
Place the electrode on the Forearm only as indicated in the manual.
•Simultaneous use of Fesia Grasp and other high frequency medical devices could cause skin
burns in the electrode area and could damage the stimulator.
•Using the system less than one meter away from shortwave or microwave therapy equipment
may cause instability in the output of the stimulator.
•Electrode placement near the thorax may increase the risk of atrial fibrillation.
•Do not attempt to repair the Fesia Grasp or open the stimulator under any circumstances. In
case of breakdown contact Fesia technician.
•Prolonged use of the electrode increases the risk of skin irritation.
•Use of worn electrodes could cause skin burns or loss of function of the system.
•Improper use or use of a defective system may cause skin burns, muscle damage or falls.
1.4 Precautions
•This device must be used under the supervision of a physician or clinician, physiotherapist or
care giver and may only be operated by personnel trained specifically for this purpose.
•The system should not be used if there is presence of lesions and/or wounds of any kind (skin,
muscle, tendon, bone...) in the area and at the time of applying electrical stimulation.
•Do not use this device if you experience any symptoms of malfunction or if any of the
components are in poor condition.
•Should not be exposed to liquids and splashes.
•Should not be exposed to extreme temperatures.
•Should not be exposed to direct sunlight.
•Should not be used near flammable products.
•Store the system under protection from moisture, dust and direct sunlight.
•Only use electrodes supplied by Fesia.
•Use only the charger and charging cable supplied and / or approved by Fesia.
Instructions for Use: Fesia Grasp
2
•This device should be kept out of reach of children.
•Caution when using the system if you have heart disease, epilepsy, or vascular or circulation
problems is recommended.
•Do not turn on the stimulator until it is properly placed on your arm.
•The electrode is personal, it should not be exchanged between different people.
•Make surethat the stimulator the sensor is charged before each use.
•The long-term effects of chronic electrical stimulation are unknown.
•The safety of using electrical stimulation during pregnancy has not been determined.
1.5 Adverse reactions
•Electrical stimulation could cause an uncomfortable feeling or very mild pain in the first usess
until the feeling becomes familiar.
•It is normal for the area where stimulation has been applied to appear red after removing the
device, this redness should disappear in about an hour.
•Electrical stimulation or gel contact with the skin may cause irritation or allergic reaction on
the contact surface in some cases.
•The patient should immediately stop using the system in the following cases:
•Redness or irritation at the site of application of stimulation for more than one hour
after removing the electrode.
•Blisters or sores in the area of application of stimulation.
•Feel a significant increase in muscle spasticity.
•Tachycardias heart feels stress or during stimulation.
•It has swelling of the forearm, wrist or fingers.
Instructions for Use: Fesia Grasp
3
2SYMBOLS AND DEFINITIONS
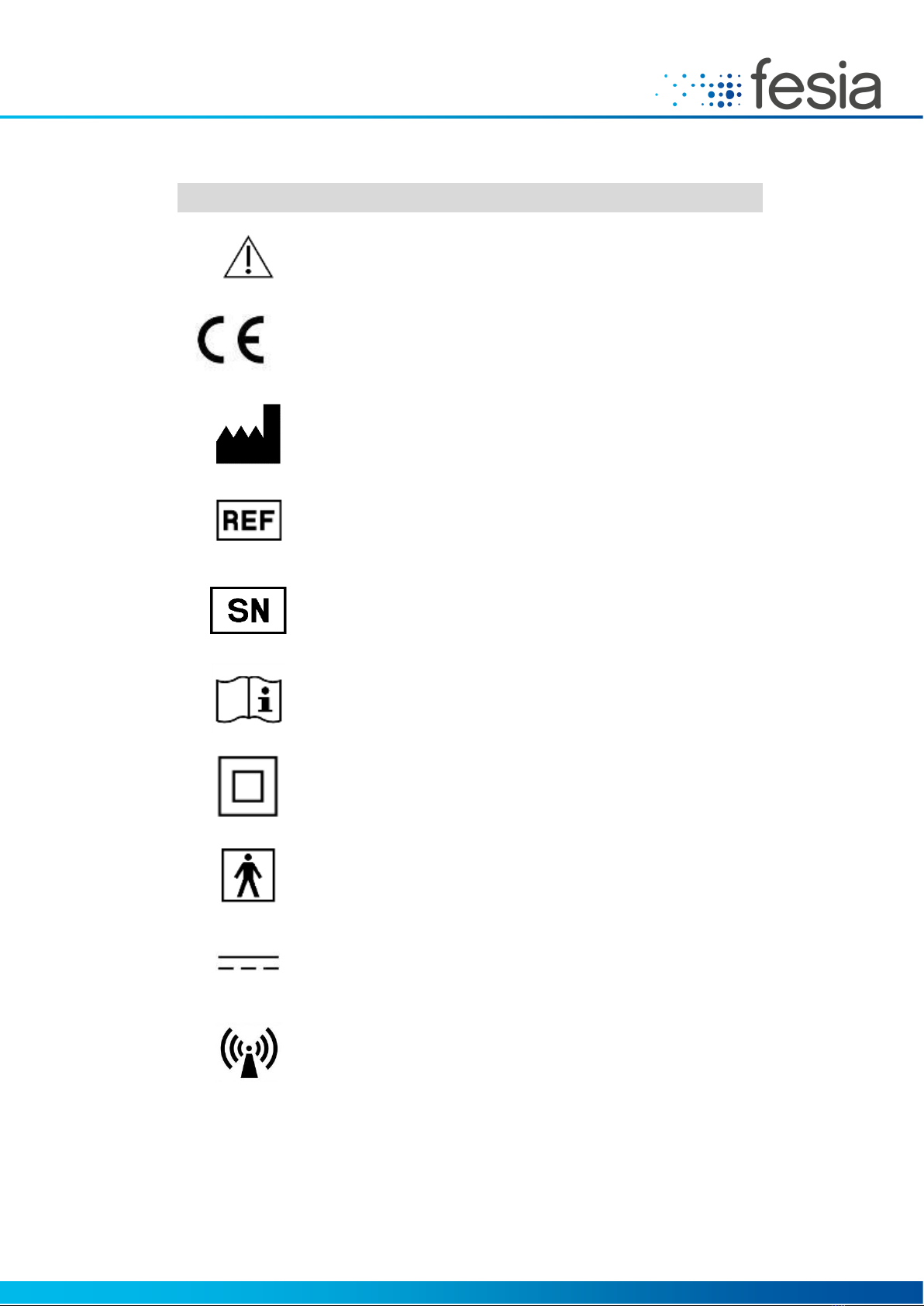
Symbol
Meaning
Caution
0051
Complies with European Medical Device Directive
Manufacturer and date of manufacture
Device reference number
Serial Number
FWxx-aassyy
xx →Device version
aass →aa (year) ss (week) of fabrication
Yy →Correlative number of the same batch
Consult the instructions
Double insulation (equivalent to Class II according to IEC 536)
Type BF applied parts
Continuous load current
Non-Ionizing Radiation
Instructions for Use: Fesia Grasp
4
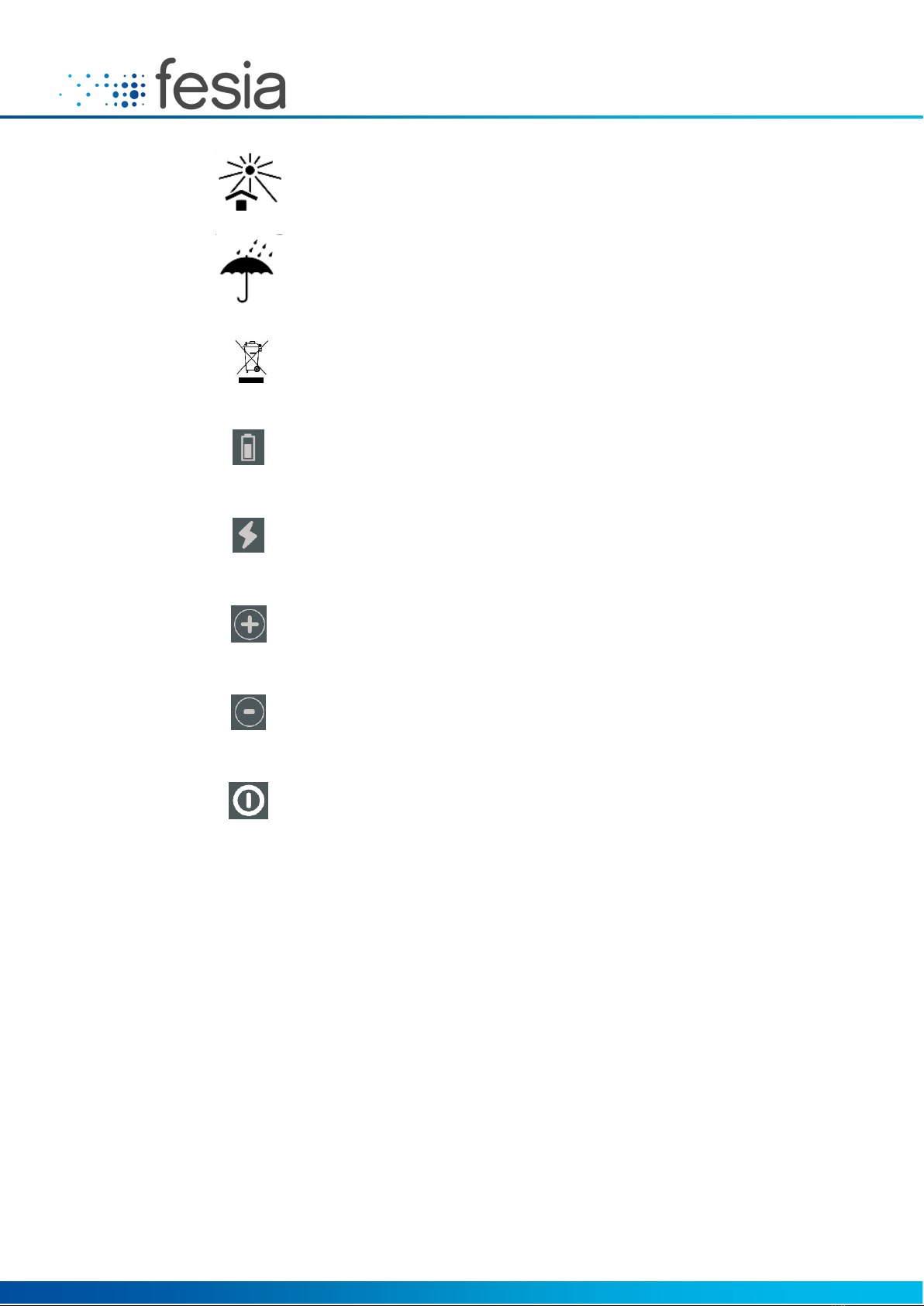
Keep out of sunlight
Keep dry
This product should not be disposed
with other household products
Indicates low battery (red light)
Indicates that the stimulation is active (yellow light)
Indicates the intensity increase button
Indicates the intensity reduction button
Indicates the button on / off system
Table 2-1: List of Symbols.

Instructions for Use: Fesia Grasp
5
3FESIA GRASP SYSTEM DESCRIPTION
3.1 General description
The Fesia Grasp device operation is based on surface electrical stimulation of the forearm muscles to provide flexion
and extension of the wrist and fingers.
Figure 3-1: Device Fesia Grasp
The main feature of this device is its multi-field electrode which allows better selectivity of movement and more natural
movements. The device consists of a stimulator, a multi-field electrode, a textile garment and a software application.
The stimulator generates electrical pulses, which are transmitted to the skin through the multi-field electrode. It is a
matrix electrode designed to cover both the posterior and lateral areas of the forearm and thus be able to stimulate the
muscles of the flexion function and extension of wrist and fingers. It consists of 32 cathodes (output fields) and 8 anodes
(return fields) that can be activated independently or in combination, thus allowing adaptation to the different patient
physiology. The multi-field electrode is personal and disposable, with an estimated life of two weeks of daily use. The
textile garment ensures proper electrode- skin contact and, on the other hand, serves as a support for both the
stimulator and electrode.
Figure 3-2: Electrode Fesia Grasp and Textile Garment

Instructions for Use: Fesia Grasp
6
Finally, Fesia Grasp has a software application that allows, on one hand, to control and configure the stimulation
parameters and, on the other, to monitor the evolution of the different patients / users in an easy and intuitive way.
The application is specifically designed for use by healthcare personnel. Chapter 5 details the characteristics of the
system.
3.2 System components
Check the case and ensure that the Fesia Grasp kit contains all of the following components:
1. Stimulator
2. Charger
3. 2 Textile garments
4. 2 Electrodes
5. Sprayer
6. Pre-configured Tablet (optional)
Figure 3-3: Components of the Fesia Grasp System
Which, in turn, contain the following elements:
Element
Battery indicator LED
Power and connectivity LED
Stimulation LED
Intensity increase button '+'
On / off button
Intensity decrease button '-'
Electrode base
Locking system
Reference of central axis
Table 3-1: Fesia Grasp Elements Description

Instructions for Use: Fesia Grasp
7
4FESIA GRASP OPERATING INSTRUCTIONS
4.1 Placement
1. Clean and dry the skin before electrode placement for optimal adhesion. The skin must be cleaned of
cream and oil residues before applying the electrode. If the area where the electrode is going to be placed
has a lot of hair, it is advisable to shave the area for hygienic reasons and to ensure proper stimulation.
2. Place the electrode on the garment. Pay attention to the laterality of the affected limb of each patient: if
working on the right side, take the electrode and the garment with indicator R (right) and L (left) if working
on the left side.
Figure 4-1: Garment structure
3. Carefully remove the protective layer that protects the gel covering the electrode. Keep this plastic, as you
will need it for storage after use. This layer serves to protect the gel from dirt and dust.
Figure 4-2: Removal of the protective layer
4. The key markers for the placement of the textile band on the forearm are the ulna and the ulnar and radial
styloid processes. The ulna should be aligned with the garment and the proximal part of the ulna should
be placed at the top of the textile band, above the indicator marked with the arrow.
Figure 4-3: Placement of the system with the central reference

Instructions for Use: Fesia Grasp
8
It is very important to leave the styloids uncovered by the textile band so that they are totally free when
closed and the wrist has total freedom of movement.
5. Then close the three parts of the textile band with the Velcro fastener.
6. Finally, insert the stimulator into the base of the electrode. Squeeze until you hear a click, and make sure
it is properly inserted.
Figure 4 5: Insertion of the stimulator
Once placed, the system should look approximately as shown in the following figure.
Figure 4-6: Fesia Grasp placement
4.2 Turning On/Off
To switch the stimulator on and off, press and hold the On/Off button for two seconds. When the stimulator has been
switched on, the power LED will start flashing. It will remain flashing until conection is stablished.
Once all system components are properly placed and turned on, use the software application described in the next
chapter to configure the system and use it while Grasping. Make sure not to use the software application more than 5
meters away from the stimulator.
Use the increase and decrease controls marked with '+' and '-' symbols respectively to adjust the stimulation intensity
level during Grasping. These changes will act simultaneously for the selected configuration.
To switch off the stimulator press and hold the on / off button for a few seconds. When the stimulator is turned off, all
LEDs will turn off.
Finally, carefully remove the garment and place the protective layer back on the electrode gel. If the electrode gel is
dry, pour a few drops of water over it before placing the protective layers on it.
Instructions for Use: Fesia Grasp
9
4.3 Luminous/acoustic indicators
4.3.1 Stimulator
Indicator
State
Meaning
Red light
Off
Suitable battery level (between 100-40%)
Slow blinking (5 sec.)
Low battery level (40-20%)
Fast blinking (1sec.)
Very low battery level (20-0%)
Continuous
Battery charging
Blue light
Off
Stimulator off
Blinking
The stimulator is waiting for connection
Continuous
The stimulator is connected to the PC
Orange light
Off
No active stimulation
Continuous
Active stimulation
Sound
Single tone short beep
Increase / decrease button pushed
Two-tone beep
Failed stimulation attempt (no current is emitted)
Table 4-1: Stimulator Indicators
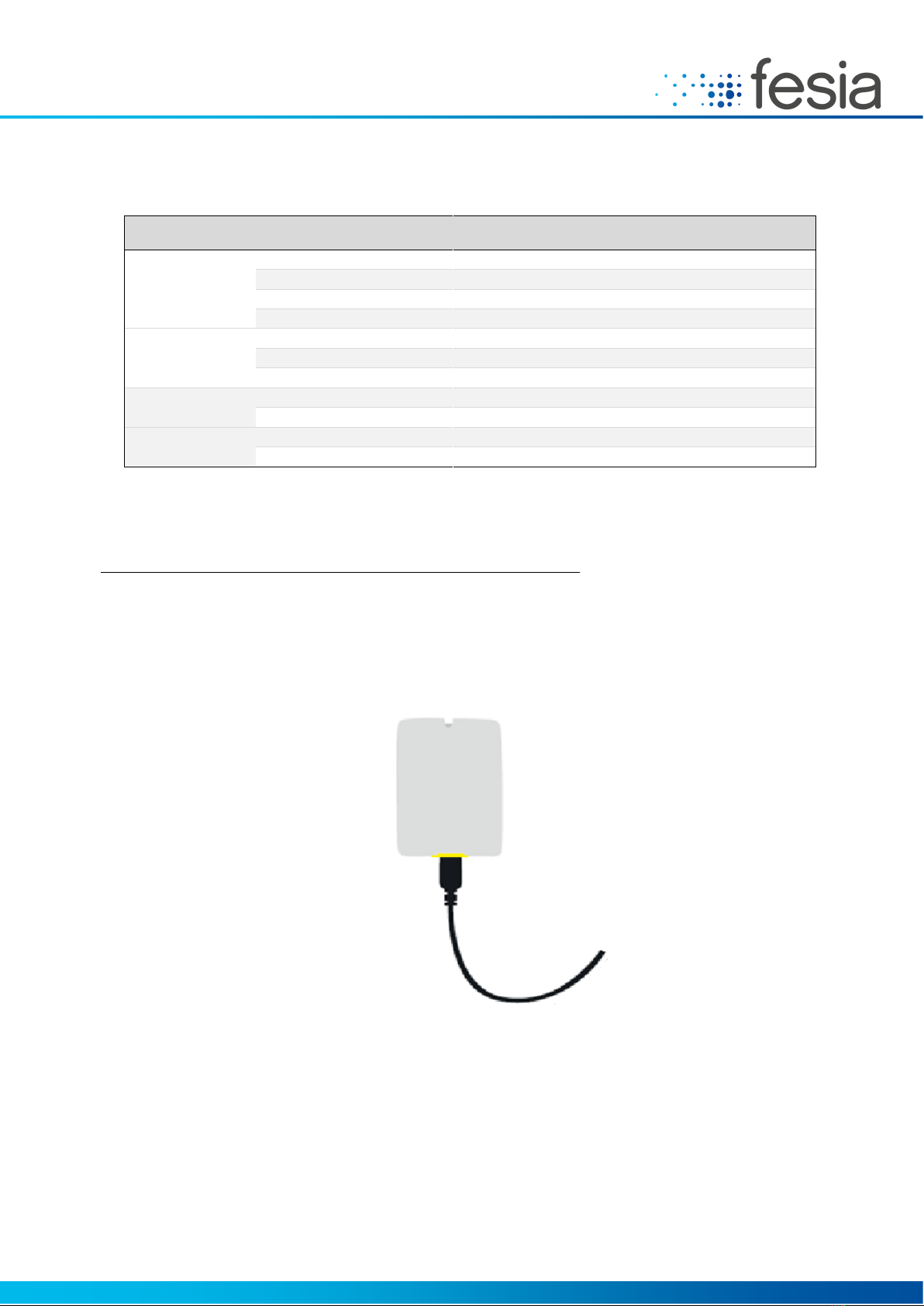
4.4 Battery charge
Use only the charger and charging cable supplied and / or approved by Fesia.
Before the first use, the batteries must be fully charged. The charging process takes about 3 hours. Connect the charger
to the network and the connector to the stimulator. Make sure that the plug is fully inserted. The red stimulator LED
will light while charging and will turn off when charging is complete.
The battery may only be replaced by authorized personnel.
Make sure both the stimulator and the tablet are fully charged before each use.
Figure 4-7: Fesia Grasp Loading
4.5 Electrode replacement
The electrode is a disposable item that should be replaced at least every 15 days of daily use or every 10-15 sessions.
However, it must be replaced if any defect or anomaly is observed. The multi-field electrodes are attached to a socket,
which is necessary for correct connection to the stimulator and to facilitate electrode replacement.

Instructions for Use: Fesia Grasp
10
Whenever the electrode needs to be changed, remove the old electrode carefully with its socket and insert the new
electrode by matching the socket in the cavity of the garment for this purpose.
Figure 4-1: Laterality indicators electrode and garment
4.6 Skin care
Lack of proper skin care and improper or prolonged use of electrical stimulation may result in skin irritation or an adverse
skin reaction. Skin irritation may occur after approximately three months of use. Therefore, it is important to follow a
daily skin care routine in order to use the system for a long time without damaging the skin.
-Clean the skin where the electrodes are attached with a damp cloth before each use. If there is presence
of oils or lotions on the skin, then wipe it off with soap and water.
-Always check for redness or rashes on the skin when placed or removed Fesia Grasp system.
-Be sure to replace the electrodes at least every 15 days and / or 10 sessions, even if they appear to be in
good condition.
-Excessive body hair in the area where the electrodes are attached may reduce skin contact. If necessary,
remove excess body hair in the place where electrodes are attached with scissors or wax. Do not use a
razor blades, as they may irritate the skin.
-When placing the garment, make sure that the electrodes make contact with the skin evenly.
-Remove the garment and electrode for at least 15 minutes every three to four hours in order to let the
skin breathe.
Instructions for Use: Fesia Grasp
11
5SOFTWARE APPLICATION
5.1 General description
The Fesia Pro application connects wirelessly via Bluetooth to the Fesia Grasp device. It is an Android application that
can be run on any tablet that meets the minimum requirements specified in section 5.2 Installation.
These are the main functions performed by the Fesia Pro application:
•Configuration of the Fesia Grasp device.
•User associated patient management.
•Monitoring of the device status (battery level, connectivity, operation mode, etc.)
It is important to ensure that the application is not used more than 50 meters away from the stimulator (in open space),
so that the connection is not lost.
If you have received a preconfigured tablet go directly to section 5.3 Login.
5.2 Installation
The device on which the application is installed must meet the following requirements:
•Android 5.1 or higher operating system
•Bluetooth connection v2.0.0 or higher
•Minimum screen size 10"
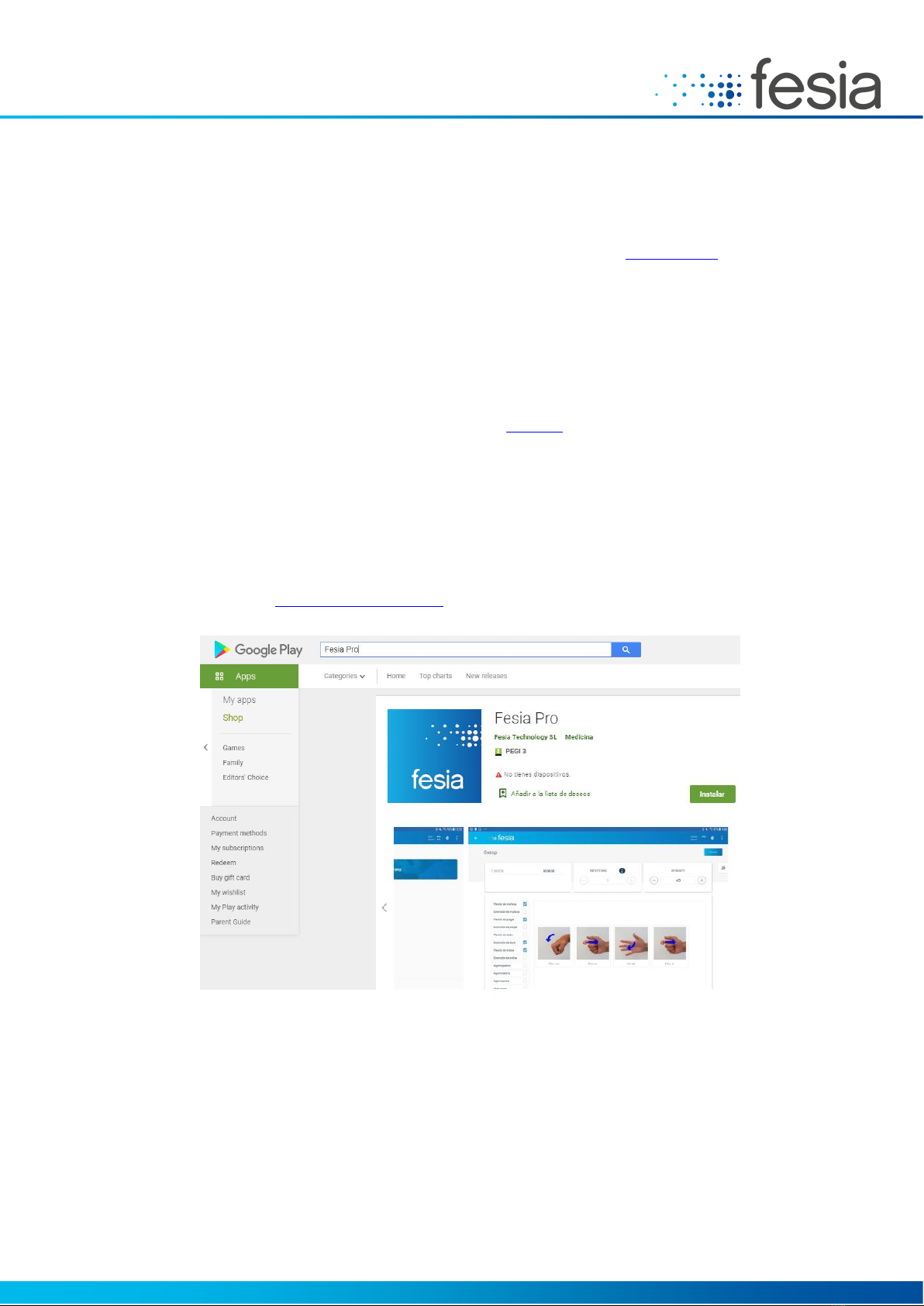
Fesia Pro is available at the Google Chrome Web Store. By searching for the application using the text "Fesia Pro" you
can access the information portal directly:
Figure 5-1: Fesia Pro application on Goofle Play
5.3 Log in
To install the application, press the "Install" option in the upper right part. The installation process will start
automatically once the consent to give permission to the application is accepted.
After a timeout, the application will appear next to the rest of the applications that the user has installed.

Instructions for Use: Fesia Grasp
12
Figure 5-2: Installed applications
5.4 Logging in
The application has user accounts to customize the operation of the application to independently manage patients and
associated devices.
Therefore, the first time the application is run, you will be asked to log in. Once the user has logged in, they will no
longer be required to log in until a logout action is performed. There is no need to log out if there is only one account
using the tablet.
Figure 5-3: Logging in screen
If you have not yet registered, you will need to create an account by clicking on "Register here".
Figure 5-4: Create new account
Instructions for Use: Fesia Grasp
13
You must fill in the requested fields. Those fields indicated with an * are the mandatory fields to be filled in. To finish
this action, click on "Create account" and the Patient Management screen will automatically open.
5.5 Bluetooth connection to the device
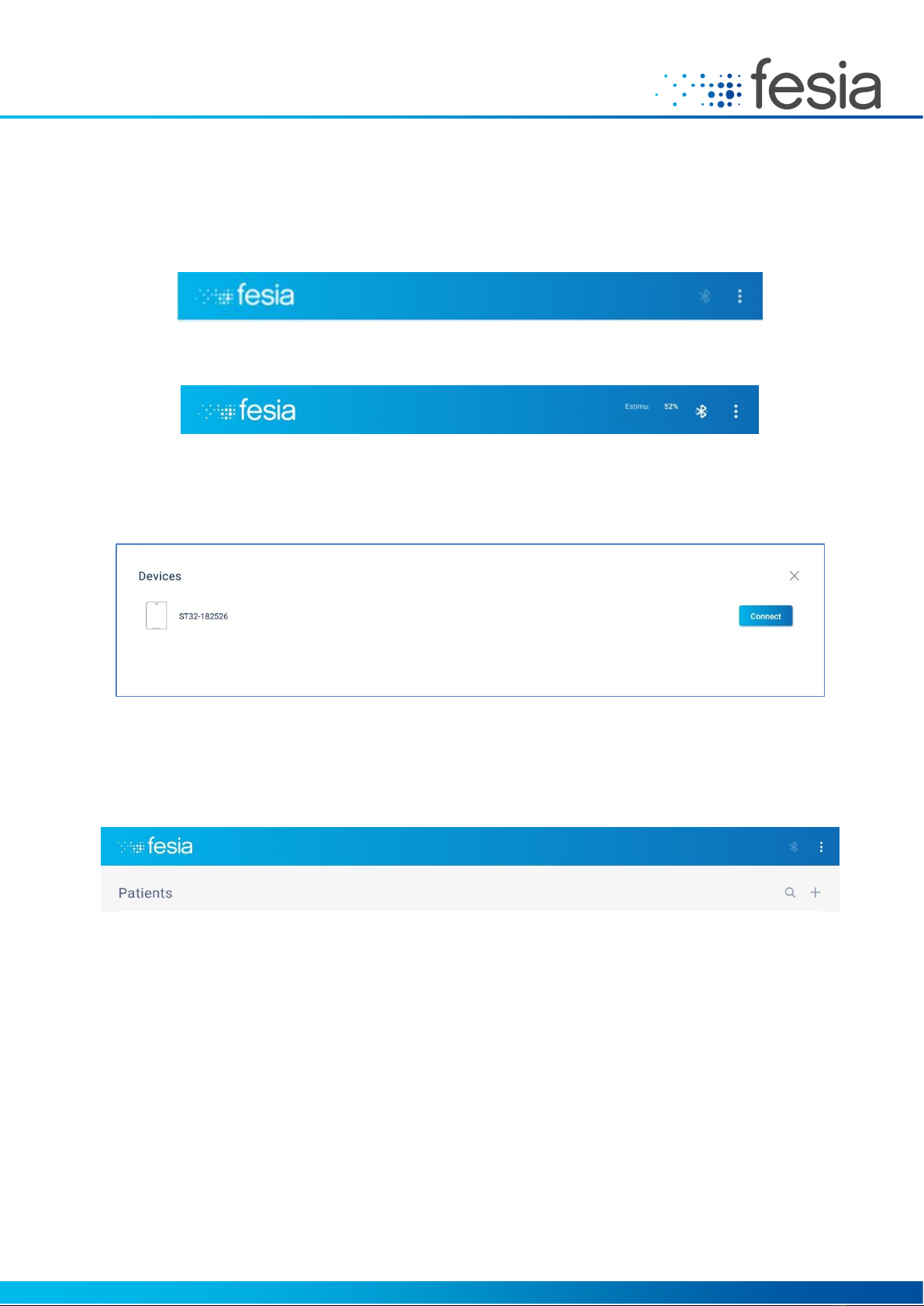
When no device is connected, no connection indication will appear:
Figure 5-5: Device no connected
When the connection is established, the battery indicator of the stimulator will appear in the header:
Figure 5-6: Device connected
5.5.1 Connecting to a device
To connect to a device, press the Bluetooth symbol. Make sure the stimulator is on. Those devices that are turned on
will be automatically listed and the "Connect" button on the device to be used must be pressed.
Figure 5-7: Connecting to a device
5.6 Patient management
5.6.1 Creating a Patient Profile
Create a Patient Profile by pressing the "+" button on the Patient Management screen:
Figure 5-8: Create a Patient profile
Then fill in the required fields and press the "Save" button. Pay special attention to select correctly the affected laterality
of the patient.

Instructions for Use: Fesia Grasp
14
Figure 5-9: Fill in patient information
5.6.2 Selecting a patient
Once created, the patient can be selected, modified or removed from the patient list.
To start a session, select the patient by clicking on his/her name.
Figure 5-10: Select a patient from the list
Once the patient has been selected, you can navigate to their data, to the sessions performed and to the rehabilitation
protocols using the buttons at the bottom of the page.
Other manuals for Grasp
2
Table of contents