Flaem Smarty Manual
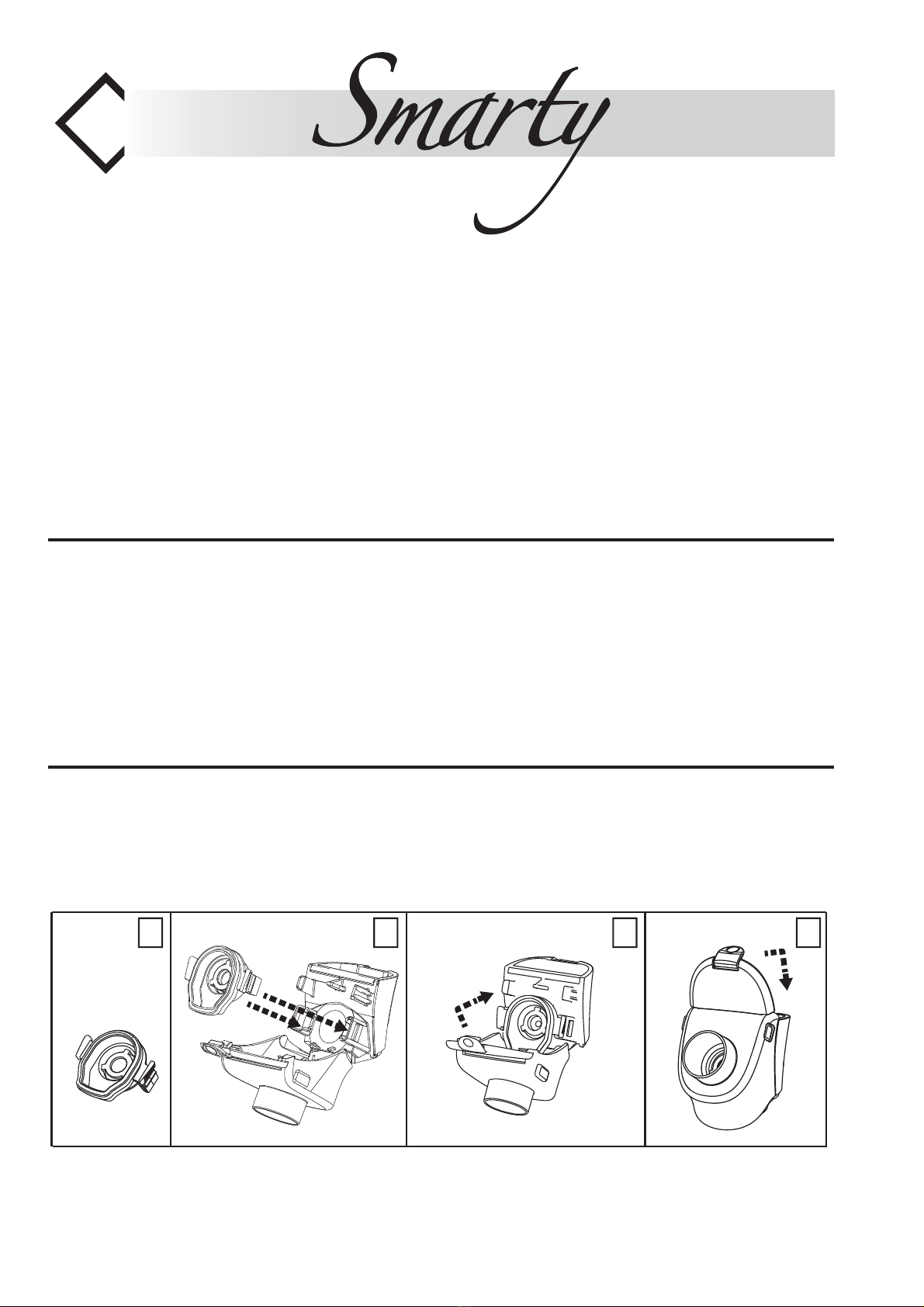
Assembly diagram - Anschlussschema -Sché-
ma de montage - Verbindingsschema - -Cro-
quis de conexiones- Schemat połączeń dla
nomenklatury
INSTRUCTIONS
FOR USE
MANUAL
MODE
D’EMPLOI
GEBRUIKS-
AANWIJZING
BEDIENUNGS-
ANLEITUNG
MANUAL DE
INSTRUCCIO-
NES DE USO
PODRĘCZNIK
INSTRUKCJI
OBSŁUGI
ENGLISH
Page 2
FRANÇAIS
Page 38 DEUTSCH
Seite 20
NEDERLANDS
Page 56
ESPAÑOL
Página 74
РOLSKI
Strona 92
2
WARNING
TO PREVENT DAMAGE TO THE MESH,
CAREFULLY FOLLOW STEPS 1 TO 4 AS LISTED BELOW
Thank you for your purchase. Our goal is the complete satisfaction of our
customers by oering cutting-edge products for the prevention and
treatment of the respiratory tract ailments.
Visit our website www.aem.it to view the whole range of Flaem’s products.
Read these instructions carefully in order to use the device correctly.
We recommend that you store this manual for future consultation.
Smarty is a medical device for home use to nebulise and administer
medication
Portable device
for aerosol therapy using
the latest in PMVT technology
(Passive MESH Vibrating Technology)
1 2 3 4
Intended use: this is an electronic Medical Device for aerosol therapy.
Smarty is suitable for nebulising medications which are either in solution
eg mucolytics, or suspension eg corticosteroids such as beclomethasone
dipropionate and budesonide) and saline solution, prescribed or
recommended by your doctor upon assessing the patient’s general
conditions. This unit may be used in medical facilities (hospitals, clinics etc.)
and at home.
For more information, a video tutorial is available at smarty.aem.it
3
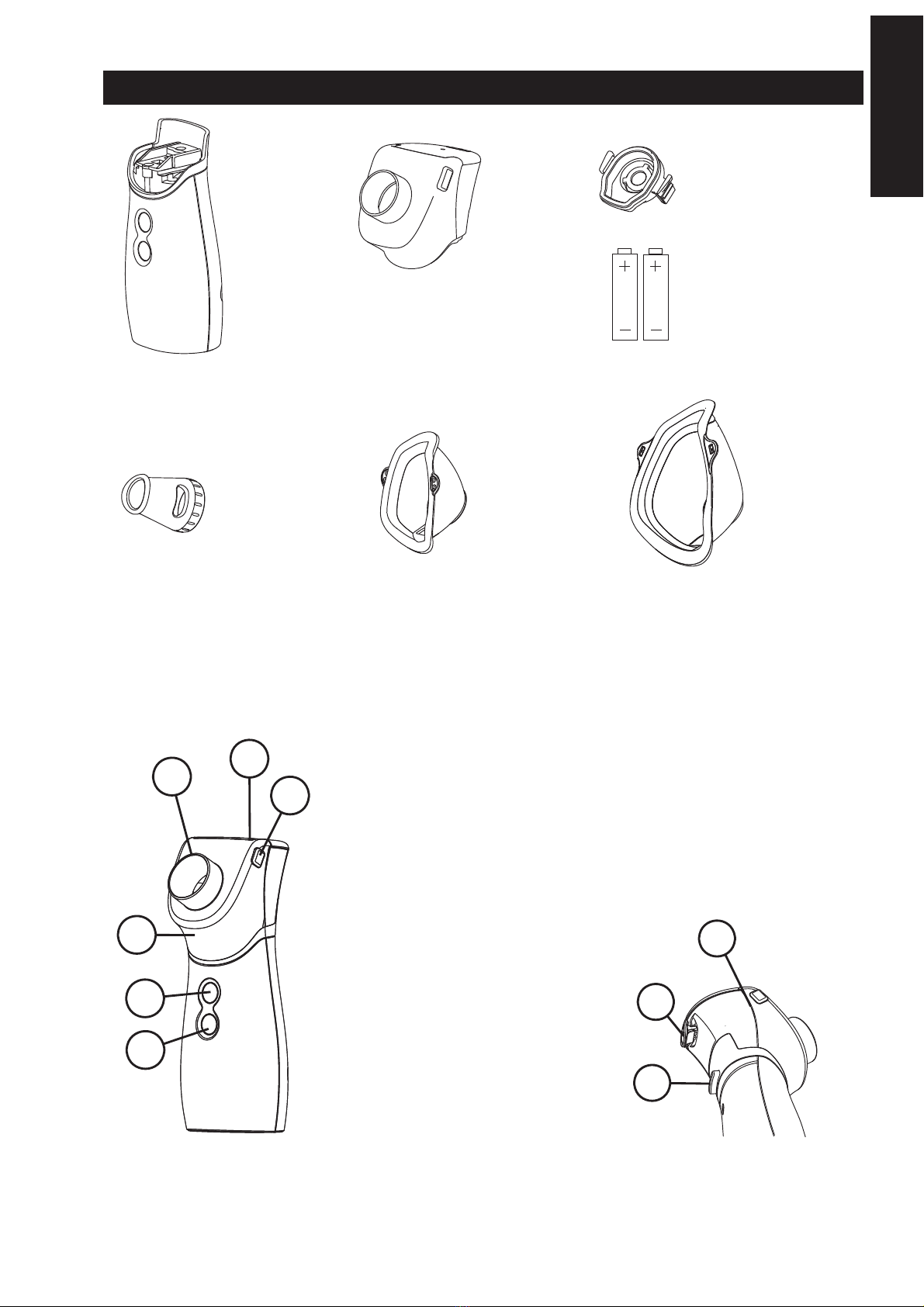
DEVICE COMPONENTS
1- Main Unit
2- Removable
medication chamber
2G- Nebuliser
head
3- Mouthpiece 4- Child mask 5- Adult mask
6 - 2 1.5V AA
alkaline batteries
2F
2E
2D
2B
1A
1B
1A - ON DEMAND nebulisation button
1B - CONTINUOUS nebulisation
START AND STOP button
1C - Medication chamber release button
2A - Medicine cup
2B - Nebulising chamber
2C - Medication cup locking clip
2D -Medication cup cover
2E - Nebulising chamber and
Medication cup release clips
2F -Accessories connection port
2A
1C
2C
ENGLISH

4
IMPORTANT WARNINGS
• As with any electrical device, especially in the presence of children, the
use of Smarty requires some basic safety precautions.
• The device should not be used as a toy. Please take particular care when it
is used by children.
• If the device is used by or in the presence of children or people who
require special assistance, the supervision of an adult who has read this
manual is necessary.
• This product must not be used by unconscious patients or not breathing
spontaneously
• Some parts of the device are small enough to be swallowed by children;
keep the device out of reach of children.
• Never use batteries other than those recommended in this manual.
• Use only batteries of known brands. Always replace both with new ones
and do not mix new and old batteries. AA alkaline or Lithium batteries
and rechargeable Ni-Mh can be used. Rechargeable batteries shall be
charged using a battery charging device that is not supplied with this
device.
• The average battery life depends on the brand used.
• It is very important to use batteries of the same brand and same type.
• Remove the batteries before storing the unit when it is not used for long
periods.
• Smarty should not be used in the presence of an anaesthetic mixture
which is ammable with oxygen or nitrous oxide.
• The device housing has been designed for limited contact with
liquids (no immersion). Do not wash the device under running water
or by immersion. Carefully follow the "CLEANING, SANITISATION,
DISINFECTION" in this manual. Absolutely do not use alcohol to clean
the device
• Do not use the device while taking a shower or a bath.
• Do not operate the device while driving or in any other situation where a
distraction could be dangerous for the user, for close people or animals or
surrounding objects.
• Do not expose the device and the batteries to extreme temperatures.
Keep the device and batteries away from heat sources, direct sunlight or
excessively hot and humid environment.
• The average duration for the device used for about 20 min a day with
medicinal products (4 applications of 5 min) is set out below:
- Main unit 5 years
- Masks and Mouthpiece 5 years
- Medication chamber 1 year
- Nebuliser head 1 year (Component subject to wear)
• Should your device fail to provide the expected performance, contact the
authorised service centre for clarications.
• Repairs must be performed by authorised FLAEM personnel only, by
complying with the information provided by the manufacturer. Any

5
unauthorized repairs will void the warranty and may pose a safety hazard
for the user.
• WARNING Do not modify this device without authorisation of the
manufacturer.
• The manufacturer, importer and seller will be held responsible for the
safety, reliability and performance, only if the device is used in accordance
with the instruction manual.
• Interactions: the materials used have been tested according to the
standards of biocompatibility (ISO 10993-5 and ISO 10993-10) in
compliance with the essential requirements of the 93/42 EEC Medical
Device Directive. The materials used in the device are biocompatible in
accordance with the provisions of Directive 93/42 EC and subsequent
amendments. However, the possibility of occurrence of allergic reactions
cannot be entirely excluded.
• If the device is used with dierent medications, it is recommended to
remove all residues. Therefore, clean it after each treatment so as to obtain
the highest degree of hygiene and to optimise the life and operation of
the device
• The device does not contain parts to be repaired by the user. The warranty
does not cover batteries or damage caused by incorrect, used up or
improperly stored batteries.
• The manufacturer should be contacted for reporting problems and/or
unexpected events related to device operation, and for any clarications
on use, maintenance/cleaning.
• Only use original accessories and spare parts by Flaem, we disclaim any
liability in the event of using non original spare parts or accessories
OPERATING INSTRUCTIONS
1 - BATTERY INSTALLATION
1.a Use a small coin to open the Battery
Compartment cover (1D) located
on the bottom of the unit.
1D

6
2 - PREPARATION
WARNING!
Before each use, the unit and the accessories should be cleaned and/or dis-
infected according to the DISASSEMBLY, CLEANING, SANITISATION, DISIN-
FECTION instructions (paragraph 5/6)
We recommend personal use, both of the nebuliser and accessories to avoid
possible risk of infection. Use only FLAEM original accessories.
2D
2C 2A
Wash your hands thoroughly before
preparing the medication
2.1 Lift the locking clip (2C) of the
Medication cup cover (2D)
2.2 Open the Medication cup cover
(2D) completely
2.3 Pour the medicine (MAX 8 ml) into
the Medication cup (2A)
2.4 Close the Medication cup cover
(2D)
2.5 Fasten the locking clip (2C)
WARNING!
After pouring the medicine into the
Medication cup (2A), the medicine
must be nebulised.
+
+
-
-
1D
6
1E
1.b Insert the two AA batteries (6),
in the Battery Compartment
(1E), making sure that the polar-
ity is correct.
1.c Close the Battery Compartment
Cover (1D) making sure that the
cover is properly.

7
3.1 Connect the desired accessory to
the Accessories connection port
(2F):
- Mouthpiece (3)
- Child mask (4)
- Adult mask (5)
If a mask is used, place it on the face as
shown in the picture
2F
5
4
3
4 - OPERATION
1A
1B
For eective therapy, after setting the appliance
up, sit in a comfortable and relaxed position and
proceed. Select the preferred delivery mode (“ON
DEMAND”or “CONTINUOUS”)
4.1 By pressing buttons (1A-1B) they light up as
follow:
FLASHING BLUE: for a few seconds, until a
complete initialization of device.
STEADY BLUE: nebulisation
FLASHING RED: batteries are used up; re-
place with new batteries of the same type
4.2 ON DEMAND button (1A) Press this button
when inhaling to obtain the delivery of medi-
cation without waste. The device only works
if you hold down the button, which will turn
blue.
4.3
START AND STOP / CONTINUOUS button
(1B): Press this button to obtain the “CONTIN-
UOUS” delivery of medication (fast treatment).
3 -
UNIT CONFIGURATION
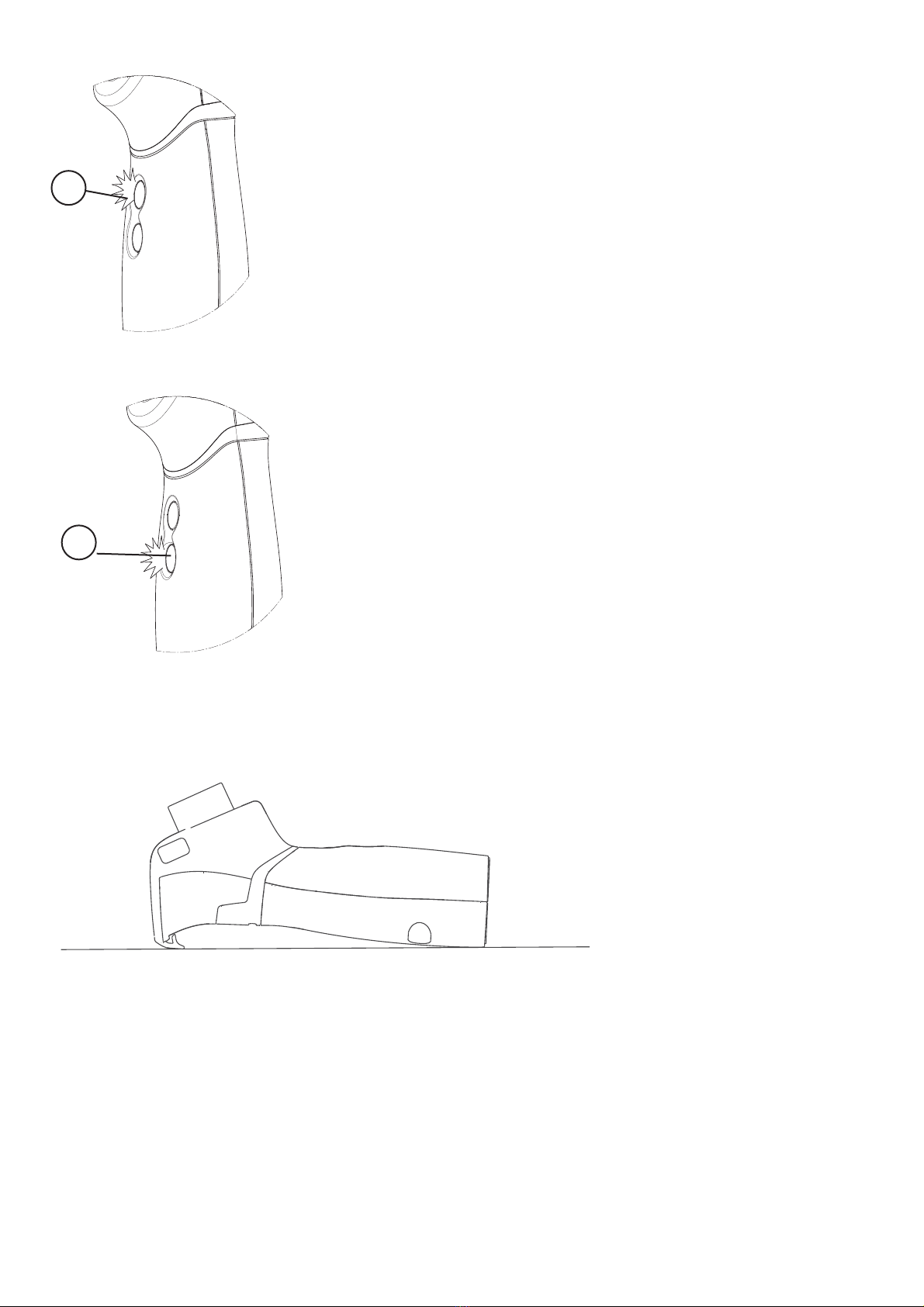
8
1A
1B
To switch o the unit, press the button (1B)
again.
4.4 Sit comfortably holding the device in your
hand, place the Mouthpiece (3) in your
mouth then breath in and out slowly through
the mouth. Alternatively, you can use the
Child Mask (4) or the Adult Mask (5).
4.5 To increase the eectiveness of treatment,
breathe slowly and deeply and after inhaling
hold your breath for a moment, so that the in-
haled medicine can deposit along the respira-
tory tract. Then exhale slowly.
4.6 The unit turns o automatically:
4.6.a after 5 minutes of use
4.6.b when, during use, the medication
chamber (2) is accidentally removed .
4.7 If you do not use the appliance for a pause
longer than 5 minutes, place it down on a
surface as shown in the picture. At the end of
each use store the device complete with ac-
cessories in a dry place away from dust.
When treatment is nished proceed as indicat-
ed in paragraphs 5 and 6.

9
5.3 To access the Nebuliser head (2G) in the Nebulising chamber(2B) re-
lease the Medication cup locking clip and open the Medication cup
cover (2D).
5.4 Press simultaneously the two release clips (2E) on both sides of the Med-
ication chamber.
5.5 Rotate until the Nebulising chamber (2B) opens completely.
5.6. Remove the nebuliser head (2G), while at the same time pressing the side
ns.
1C
2
2E
2E
2B
2D
2C
PUSH
PUSH
PUSH
2C
2E
2D
2E
2B
5 - DISASSEMBLY
When treatment is nished:
5.1 Press the Medication chamber release button (1C) positioned on the
back of the unit.
5.2 Remove the Medication chamber (2).
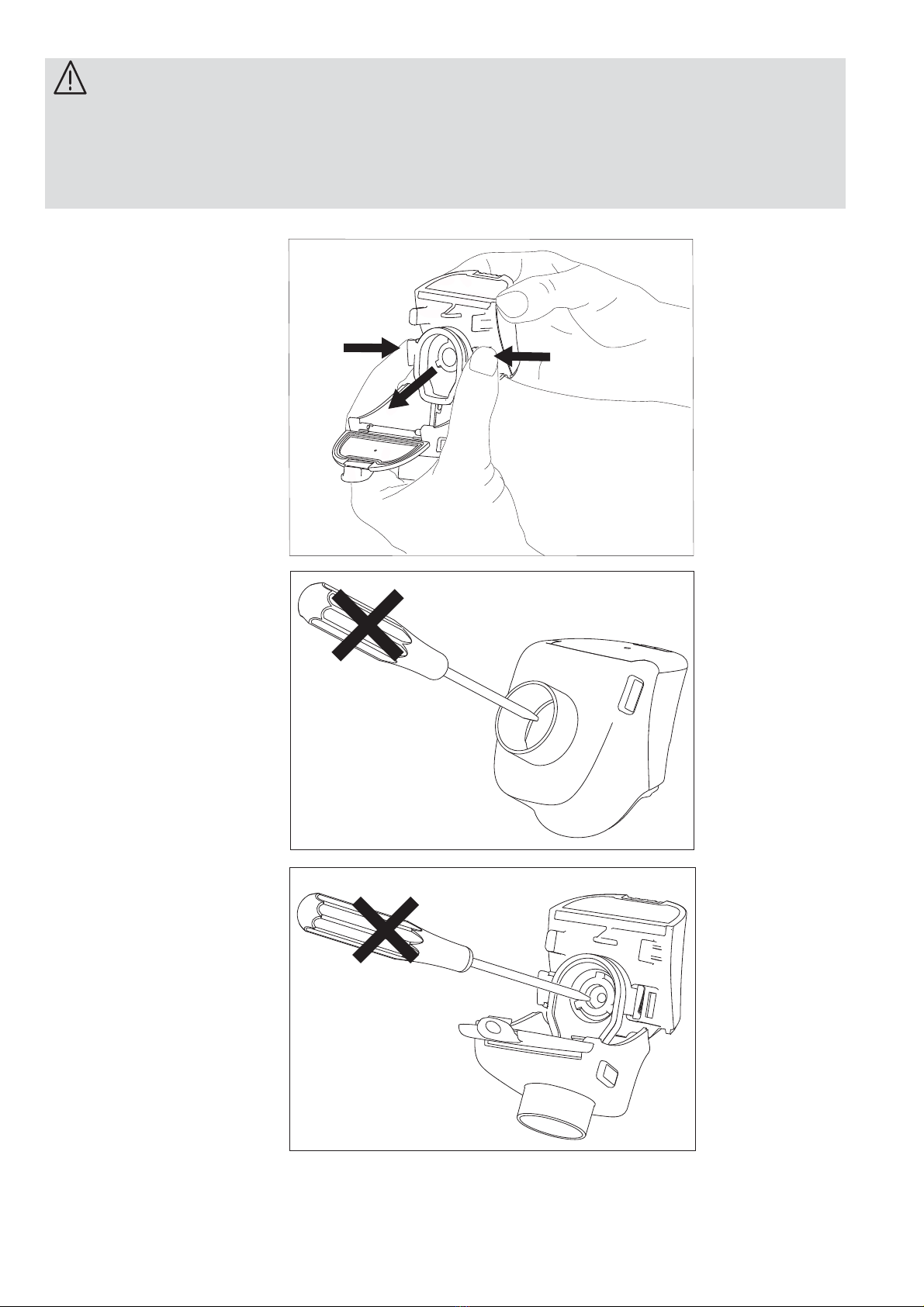
10
WARNING
• Do not use tools: this may cause irreparable damage to the Nebuliser
head (2G).
• Do not press the centre of the Nebuliser head (2G) with your ngers or
tools from the inside: this may cause irreparable damage.
OK
5.7 Carefully clean it according to the CLEANING, SANITISATION, DISIN-
FECTION instructions in paragraph 6.

11
6 - CLEANING, SANITISATION, DISINFECTION
Before and after each use, the device and the accessories must be
cleaned and disinfected properly as described below. If this is not done,
some microorganisms may collect in the device, causing the risk of in-
fection. Do not use alcohol or other solvents to clean the device.
6.1. CLEANING Accessories and Removable Upper Part (2):
6.1.1. WASHING: Wash the components 2-2G-
3-4-5 with lukewarm drinking water with dish
washing soap (non-abrasive), then rinse thor-
oughly with a jet of lukewarm drinking water
to remove any detergent residue.
6.1.2. DRYING
6.1.2.a. After washing and rinsing all the com-
ponents, shake them to remove excess water
and reassemble them, as described in the fol-
lowing paragraph 7. REASSEMBLY OF THE
DEVICE. At this point, to eliminate the resid-
ual water that has deposited in the nebuliser
head (2G), press Button 1B (side gure) and
wait for the device stop to nebulizing. Turn
o the unit. This operation is very important,
because it prevents the deposit of limestone
in the micropores of the Nebuliser Head (2G)
which could aect proper nebulisation of the
drug in subsequent therapeutic applications.
6.1.2.b. To complete the drying of all the compo-
nents, leave them out and in a dry place (e.g.
not in the bathroom) as into the side gure.
WARNING
Do not clean the Mesh (2G) using brushes or
other instruments: this may cause irreparable
damage.
2G
2
3
5
4
5
2
2G
3
4
PUSH

12
WARNING
Do not immerse the Main Unit (1) in water and
do not wash it under running water.
6.2 CLEANING the Main Unit
To clean the Main Unit(1) use a cloth slightly moistened
with an antibacterial detergent, then dry it with a soft
paper towel.
6.3. SANITISATION
6.3.1. Immerse for at least 30 minutes the
opened Removable Medication Chamber
(2) and the accessories 2G-3-4-5, in a solu-
tion of 50% water and 50% white vinegar,
then rinse thoroughly with hot running wa-
ter.
6.1.2. DRYING
6.1.2.a. After washing and rinsing all the com-
ponents, shake them to remove excess wa-
ter and reassemble them, as described in
the following paragraph 7. REASSEMBLY
OF THE DEVICE. At this point, to eliminate
the residual water that has deposited in the
nebuliser head (2G), press Button 1B (side
gure) and wait for the device stop to nebu-
lizing. Turn o the unit. This operation is very
important, because it prevents the deposit
of limestone in the micropores of the Nebu-
liser Head (2G) which could aect proper
nebulisation of the drug in subsequent ther-
apeutic applications.
6.1.2.b. To complete the drying of all the com-
ponents, leave them out and in a dry place
(e.g. not in the bathroom) as into the side
gure.
2G
2
3
5
4
5
2
2G
3
4
PUSH

13
6.4.1 METHOD A: DISINFECTION BY STEAM
6.4.1.a
Disinfect the accessories provided in
Table 1 following the disinfection procedure
with device for feeding-bottle (without mi-
crowave), it requires a treatment duration
of at least 15 minutes. It is necessary to use
demineralised or distilled water to prevent
limestone deposits, which could impair the
function of the nebuliser head. Carefully
read the instructions for use of the device
in question, paying special attention to the
amount of needed water and the method
to be followed to carry out the disinfection
phases.
6.4.2 METHOD B: DISINFECTION BY BOILING
6.4.2.a
Disinfect the accessories provided in
Table 1 by boiling for 20 minutes; it is neces-
sary to use de mineralized or distilled water
to prevent limestone deposits, which could
impair the function of the nebuliser head.
6.4.2.b DRYING:
Before making a new ap-
plication wait until the components have
cooled down and dried.
6.4.3
METHOD C: DISINFECTION BY CHEMICAL
WARNING Except the nebuliser head
2G
6.4.3.a
The disinfectant to be used must be an
electrolytic chloroxidizer (active principle:
sodium hypochlorite), specic for disinfect-
ing, which is available in any pharmacy.
6.4.3.b
Fill a container, whose size is suitable
to hold all of the accessories to be disinfect-
ed listed in Table 1, with a drinking-water
and disinfectant solution, according to the
proportions indicated on the packaging of
the disinfectant itself.
6.4.3.c
Completely immerse each accessory in
the solution, taking care to avoid the forma-
tion of air bubbles that may come in contact
with the accessories. Leave the accessories
immersed for the amount of time indicated
on the packaging of the disinfectant, and
as
sociated with the concentration chosen
to
prepare the solution.
6.4.3.d
Remove the accessories and rinse
DO NOT IMMERSE IN
SOLUTIONS BASED ON
CHLORINE

14
7 - DEVICE RE-ASSEMBLY
7.1. Insert the Spraying Head (2G) in its
seat on the front of the medicinal
product container until the double
click sound indicates successful cou-
pling.
WARNING
Do not use any tools, you could damage the head.
method a) method b) method c) method d)
Equipment bot-
tle disinfection
by steam (not
a microwave
oven)
Boiling with
demineralised
or distilled
water
Electrolytic
chloroxidizer Alcohol
2G
Nebulizer head YES YES NO NO
3
Mouth piece YES YES YES YES
4 –5
Adult and Child
mask YES YES YES YES
2
Complete medica-
tion chamber
(2G excluding)
YES YES YES NO
DISINFECTABLE
PARTS
METHODS
them with plenty of lukewarm drinkable
water.
6.4.3.e DRYING:
carefully perform all the
operations described in paragraph 6.1.2.
of the user manual.
6.4.3.f
Dispose of the solution following the
instructions provided by the disinfectant
manufacturer.
2
5
4
3
5
2
3
4

15
7.3. Close the complete pull-out
medicine container (2) by rotating
the Front Part (2B) towards the
Drug Container (2A) until the two
Container Buttons (2E) are hooked.
7.4. Close the Medication Cup Cover
(2D) by means of the locking clip
(2C)
7.5. Insert the Removable Medication
container (2) onto the Main Unit
(1) matching the guides on the two
parts.
7.6. Push the Removable Medication
container (2) right down until the
Medication container release
button (1C) located in the rear of
the Main Unit (1) springs with a
CLICK.
2E
2E
2E
2E
2D 2C
1
2
CLICK
1
1C

16
TROUBLE-SHOOTING
If after checking the aforementioned conditions, the unit still does not nebulise, we rec-
ommend that you contact the dealer or an authorised Flaem service centre.
PROBLEM CAUSE SOLUTION
The device
switches on but
does not nebulise
or nebulises little.
• Malfunction of the
Mesh (2G)
• Soak the Nebulising head
(2G) in a solution of water
(50%) and white wine vinegar
(50%), rinse and t back on as
described in paragraph 6.3
SANITISING
• The Nebuliser head
(2G) is broken
• Replace it with a new Nebuli-
ser head (2G)
• No medicine supply
in the nebulising area
• Verify that the small hole in the
centre of the Medication cup
cover (2D) is not obstructed.
If so clean it with a needle
• The nebulising speed
depends on the type
of medicinal product
used
• The inhaling treatment time
depends on the type of
medicinal product and on the
patient’s inhaling ability
The device
switches o after a
few seconds (blue
light).
• No electrical contact
between the Remov-
able Medication
chamber (2) and the
Main Unit (1)
• Check that the Removable
Medication chamber (2)
is properly hooked to Main
Unit (1)
The device
switches
o
• It’s been 5 minutes
since device switch-
ing on • Turn on the unit again
The START AND
STOP Button (1B)
shows a ash-
ing red light and
the device does
not nebulise or
switches o.
• Batteries are used
up or low
• Replace the batteries if they
are alkaline.
• Recharge the Batteries if they
are rechargeable Ni-MH or
Lithium type.
The device does
not switch on. • No electric power
supply
• Verify that Batteries are insert-
ed with the correct polarity.
• Used up or low Batteries.
Replace or recharge.

17
8 - TECHNICAL CHARACTERISTICS
9 - OPERATING CONDITIONS
10 - STORAGE CONDITIONS
Smarty Electronic Nebuliser Model P0315EM
Power Supply 3.0Vdc with 2 Batteries
AA Alkaline, 1.5Vdc
2.4Vdc with 2 Batteries
AA Rechargeable Ni-MH or Lithium, 1.2Vdc
Power 1.5W
Transducer frequency 170 KHz
Noise level (at 1 m) less than 20 dBA
Device dimensions 54(W) X 69(D) X 140(H) mm
Device weight (excluding batteries) 125g
Carry bag dimensions 140(W) X 80(D) X 150(H) mm
Delivery rate ml/min(1) 0.25 ml
Characterization *MMAD 4,00 m approx
Breathable fractions < 5 m (FPF): 65% approx
Medication cup capacity(1) 8 ml
(1) The nebulising speed has been measured with a 0.9% saline solution at 23° C according to the
internal Flaem procedure I29-P07.5. It may vary according to the nebulising head supplied, medicinal
product and environmental conditions. Values shown for the aerosol delivery may also vary depending
on the patients respiratory Capacity.
* Please visit Smarty.Flaem.it or contact Flaem Nuova S.p.A Via Colli Storici, 221 25015 S. Martino della
Battaglia (BS) Italy - Tel +39 030 9910168 for information concerning nebulisation characteristics.
APPLIED PARTS
The BF type applied parts are: patient accessories (3,4,5)
Temperature min 10°C max 40° C
Air humidity min 10% max 75% RH
Atmospheric Pressure min. 690 hPa max. 1060 hPa
Temperature min 10°C max 35°C
Air humidity min 10% max 75% RH
Atmospheric Pressure min. 690 hPa max. 1060 hPa
If the appliance is stored at a temperature other than indicated under the
storage conditions, leave the device at room temperature for at least 1 hour
prior to use. With medicinal products in suspension or particularly viscous,
the information provided according to standard EN13544-1 might be subject
to changes.
Temperature, atmospheric pressure and humidity might aect the appli-
ance’s performance.

18
11 - SYMBOLS
0051
Warning
Type BF applied parts
WARNING! Check the
instructions for use manual
Direct current
CE Marking medical ref.
Dir 93/42 EEC Directive and
subsequent updates
Battery disposal: Used up
batteries must be disposed
of in appropriate containers.
Manufacturer
Keep dry
Serial number
of the device
Positive batteries polarity
Negative batteries polarity
12 - DISPOSAL OF DEVICE
13 ELECTROMAGNETIC COMPATIBILITY
In conformity with Directive 2012/19/EC, the symbol shown on the
device to be disposed of indicates that it is considered as waste and is
therefore subject to “sorted waste collection”. The user must therefore
take (or have taken) the above waste to a pre-sorted waste collection
centre set up by the local authorities, or else give it back to the dealer when
purchasing a new appliance of the same type. Pre-sorted waste collection
and the subsequent treatment, recovery and disposal operations favor the
production of appliances made of recycled materials and limit the negative
eects of any incorrect waste management on the environment and public
health. The unlawful disposal of the product by the user could result in
administrative nes as provided by the laws transposing Directive 2012/19/
EC of the European member state or of the country in which the product is
disposed of.
This device has been designed to satisfy requirements currently required
for electromagnetic compatibility (EN 60 601-1-2). Electrical medical
devices require special care during installation and use with respect to
EMC requirements. It is therefore required that they be installed and/
or used according to the manufacturer’s specication. Potential risk
of electromagnetic interference with other devices. Radio and mobile
telecommunications devices or portable RF (mobile phones or wireless
connections) may interfere with the operation of electrical medical devices.
For further information visit www.aemnuova.it website. The Device may be
subject to electromagnetic interference if other devices are used for specic
diagnosis or treatments. Flaem reserves the right to make technical and
functional modications to the product without notice.
19
AVAILABLE SPARE PARTS AND ACCESSORIES
Mouthpiece
(made of silicone) ACO563P
Child mask
(Soft-touch silver) ACO438P
Adult mask
(Soft-touch silver) ACO437P
Nebuliser head ACO655P
Complete removable
medication container ACO656P
14 EXPECTED DURATION
Expected duration: The service life of the various components set out below
refer to using the device for about 20 min a day (4 appli-
cations of 5 min.)
Main unit 5 years
Masks and Mouthpiece 5 years
Medication chamber 1 year
Nebuliser head 1 year
(Component subject to wear)
20
ACHTUNG
UM SCHÄDEN AM ZERSTÄUBUNGSKOPF ZU VERMEIDEN,
SORGFÄLTIG DIE UNTEN ANGEGEBENE REIHENFOLGE VON 1 BIS 4 AUSFÜHREN.
Vielen Dank, dass Sie unser Produkt erworben haben. Unser Ziel ist die
Zufriedenheit unserer Kunden, indem wir ihnen innovative Produkte zur
Vorbeugung und Behandlung der Atemwege anbieten. Informationen zu
unseren Produkten nden Sie auf der Website www.aem.it
Lesen Sie diese Anleitung für eine korrekte Anwendung des Gerätes
aufmerksam durch.
Wir empfehlen, diese Anleitung für eventuelle weitere Beratungen
aufzubewahren.
Tragbares Gerät
Für Aerosoltherapie
mit PMVT-Technologie
(Passive MESH Vibrating Technology)
1 2 3 4
Zweck des Gerätes: Dieses medizinische Vorrichtung ist ein Gerät für die
elektronische Aerosoltherapie und dazu geeignet, Medikamente in Lösung
(normalerweise Mukolytikum), Medikamente in Suspension (z.B. Corticosteroide,
wie Beclometason und Budesonid) und physiologische Lösung , die vom Arzt, der
den allgemeinen Zustand des Patienten untersucht hat, verschrieben bzw. empfohlen
wurden, zu zerstäuben.
Diese Einheit kann in medizinischen Einrichtungen (Krankenhäuser, Kliniken usw.) und
in häuslicher Umgebung verwendet werden.
Weitere Informationen nden Sie in einem Video-Tutorial unter folgendem
Link: smarty.aem.it
Other manuals for Smarty
2
This manual suits for next models
3
Table of contents
Languages:
Other Flaem Personal Care Product manuals
Popular Personal Care Product manuals by other brands
Otto Bock
Otto Bock 1S101 SACH+ Instructions for use

Ace Innovation
Ace Innovation SuperVisor Assembly & user instructions

Sensio
Sensio Isla installation instructions

Pebble Grey
Pebble Grey GLENDALE 800.10.30 Assembly instructions

Remington
Remington CB7408 manual

KEUCO
KEUCO 17612 01 9051 Installation and operating instructions