Forest Dental Syringe Manual

3.16.19 0097-407 Rev. A 4 of 4 1 of 4
Forest Syringe Reprocessing and Maintenance Operator’s Guide
Aordable Excellence + Designer Friendly®
Indications for Use/Intended Use e Forest Dental Units are intended to serve as a base for ancillary dental devices and
accessories by providing air, water, vacuum, and low voltage electrical power to hand-held dental instruments. e Forest Dental
Units are intended for use by dental practitioners to provide diagnostic and therapeutic treatment to dental patients in a clinical
environment. ere are no contraindications for this product.
Conformity Assessment:
Medical Device Directive Annex V
Refer to Forest General Information Operator’s Guide for Glossary of Symbols, Terms,
EMC/Electrical Safety Declaration and User Guidance.
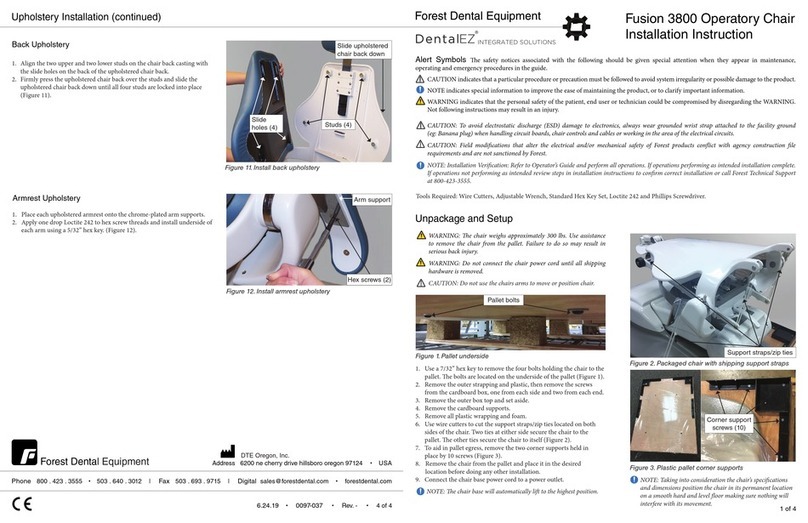
Quick Release Syringe Assembly 1109-010 Forestar-3 Syringe Assembly 1107-086
Overview
Syringe head
Syringe body
Push collar
Barrel adapter
Syringe head
Syringe body
Changing the Tip: Changing the Tip:
To remove the syringe tip, press down on
the large collar. When you feel a so “click”,
the tip may be pulled straight out.
To insert the syringe tip, hold the collar
down and insert the new sterilized tip.
Be sure to press it all the way in, and
then release the collar.
To remove the syringe tip, rmly grasp tip
and pull away from syringe head.
To insert the syringe tip, push the new
sterilized tip into the barrel adapter until
you feel the barrel engage with the tip.
e Centers for Disease Control and Prevention’s (CDC) recommendations for the cleaning, disinfection, and sterilization
of dental equipment can be found in:
Guidelines for Infection Control in Dental HealthCare Settings – 2003 www.cdc.gov/mmwr/PDF/rr/rr5217.pdf
Guidelines for Disinfection and Sterilization in Healthcare Facilities – 2008
www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines.pdf
Summary of Infection Prevention Practices in Dental Settings – 2016
https://www.cdc.gov/oralhealth/infectioncontrol/pdf/safe-care.pdf
e American Dental Association’s recommendations can be found at: www.ada.org
e Organization for Safety and Asepsis Procedures recommendations can be found at: www.osap.org
FDA Guidelines for Dental Unit Water Line Maintenance can be found at: www.fda.gov
Resources
WARNING: Test the installation of syringe tip by giving a pull on the tip to assure that it is inserted all the way and that the locking
mechanism is fully engaged.
WARNING: Forest syringe tips must be used with Forest syringes. Forest does not authorize the use of syringe tips from any other
source.
DTE Oregon, Inc.
Address 6200 ne cherry drive hillsboro oregon 97124 USA
Phone 800 . 423 . 3555 503 . 640 . 3012 | Fax 503 . 693 . 9715 | Digital [email protected] forestdental.com
Forest Dental Equipment

2 of 4 3 of 4
1. Aer patient use, run syringe water for 30 seconds to help prevent cross-contamination between patients.
2. With gloved hands, remove used syringe tip (refer to page 1 for tip removal) for sterilization and dispose of syringe
barrier protection.
3. Use clean gloves to install replacement barrier protection on syringe body/head (refer to page 1 for part identication)
and sterilized syringe tip with each new patient.
WARNING: Forest syringes (excluding syringe tip) must be operated with FDA-cleared barrier protection (FDA Product Code: PEM)
during a procedure following Forest’s instructions; Forest syringe tip must be sterilized between patients.
1. Syringe tip must be sterilized before initial use and in between patients using the following parameters.
NOTE: e Syringe aluminum body and head remain on the tubing (refer to page 1 for part identication).
CAUTION: Be sure to rinse all pre-autoclave cleaning agents from components prior to sterilization.
WARNING: Sterilization parameters are gravity displacement steam autoclave. Parameters for wrapped (Sterilization Wrap FDA
Product Code: FRG) are 132°C (270°F) for 15 minutes at temperature with a 30-minute drying time. Parameters for unwrapped
(ash steam sterilization) are 132°C (270°F) for 3 minutes at temperature with a 30-minute drying time. Syringe tip weight:
0.224 oz
2. If visual inspection shows barrier has been physically compromised or barrier did not adequately cover syringe head or
body, immediately clean and then disinfect syringe body, head and tubing (refer to page 1 for part idenication).
3. When properly using barrier protection, clean and disinfect syringe body, head and tubing at the end of day following End
of Day procedure.
Reprocessing Procedure: End of Day, Between Patients when Barrier is Compromised and High-Risk
Patients
1. Dental unit can remain on. Aer syringe tip is removed for sterilization (refer to page 1 for tip removal) using a product
labeled as a cleaner (or a solution of mild dish soap/warm water) applied to a wipe or so paper towel, clean contact
surfaces and remove visible soil/debris and contaminants from syringe body, head and syringe tubing (refer to page 1 for
part identication).
2. Detach (by unscrewing) the syringe head from the syringe body (both remain on tubing).
3. Disinfect syringe body, head and syringe tubing using an intermediate-level EPA-registered disinfectant (excluding
chlorine-based products) with the intended use in healthcare facilities (such as CaviWipesTM). Be sure to wipe around
the threads and inside of the parts. Slide syringe body down tubing as necessary to adequately expose all areas to
disinfectant.
4. Contrary to disinfectant label instructions for dwell time, allow disinfectant to remain wet on parts for at least a 4-minute
dwell (contact) time. Increased dwell time is due to various material and porosity types of Forest units. Use multiple
wipes to adequately wipe each surface and in order to keep surface visibly wet for the entire time. Allow surface to air dry
for no less than 1 minute.
5. Aer one minute if still wet, wipe surface dry and then repeat step 4 to allow for adequate disinfection due to various
material and porosity types.
6. Remove disinfectant residue with a solution of mild dish soap/warm water and wipe dry to minimize the harmful eects
of chemical disinfectant residues. Not doing so will accelerate degradation of materials over time.
7. Reattach syringe head to syringe body (by screwing).
Reprocessing Procedure: Between Patients
Procedure: Between Patients Replace Barrier Protection and Syringe Tip
Forest Air/Water Syringe and Syringe Tip
WARNING: Always wear gloves when handling contaminated components of the dental unit including used barrier protection and
while cleaning, disinfecting and sterilizing clinical contact surfaces. Be sure to change gloves aer handling contaminated
material or devices.
WARNING: Forest syringes (excluding syringe tip) must be operated with FDA-cleared barrier protection (FDA Product Code: PEM)
during a procedure following Forest’s instruction for use; Forest syringe tip must be sterilized between patients.
WARNING: Even with the use of barrier protection, daily clean and then disinfect clinical contact surfaces with an
intermediate-level EPA-registered disinfectant (excluding chlorine-based products) with the intended use in healthcare
facilities and adhere to manufacturer’s instruction for use.
WARNING: Always consult the instruction for use and the Safety Data Sheet (SDS) of the disinfectant manufacturer to be aware of
any hazards.
CAUTION: Disinfectant products should not be used as cleaners unless the label indicates the product is suitable for such use.
Ensure the intermediate-level disinfectant product is compatible with the surfaces to which it is being applied. With any disinfectant,
daily cleaning with mild dish soap/warm water and wiping dry is required to minimize the harmful eects of chemical disinfectant
residues.
CAUTION: Forest makes no warranty, expressed or implied, that the use of disinfectants will not damage the surface nish
of the equipment. Damage and discoloration of the surface nishes due to chemical disinfection is not covered under warranty.
CAUTION: Do not use powdered cleansers or abrasive scrubbers on any dental unit surface. To remove dried-on material, use a
so-bristled brush.
WARNING: Follow all local, State and Federal regulations for proper infection control. e Centers for Disease Control and Prevention’s
recommendations for the cleaning, disinfection, and sterilization of dental equipment: Visit www.cdc.gov “Guidelines for Infection
Control in Dental Health-Care Settings — 2003” and “Summary of Infection Prevention Practices in Dental Settings” as well as
e American Dental Association’s recommendations can be found at: www.ada.org.
For questions or additional information on reprocessing your Forest equipment, please contact Forest Technical Support at
800-423-3555.
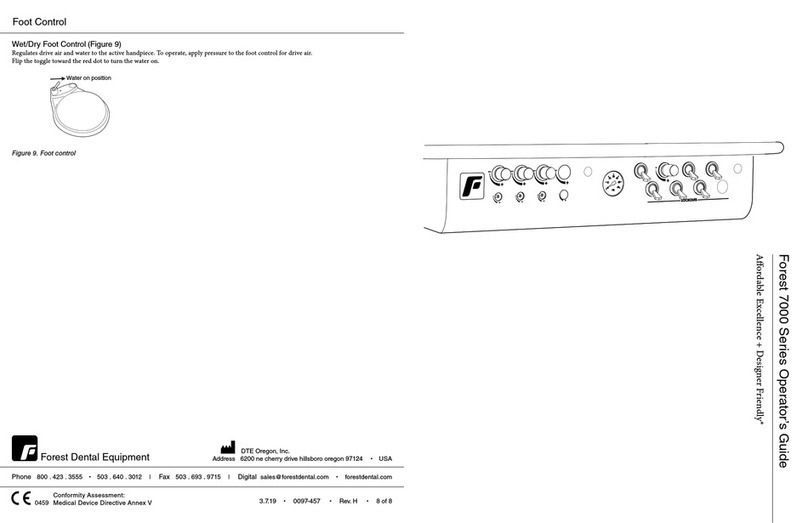
e syringe is an instrument powered by a dental unit intended to supply air and water to the oral cavity of dental patients. Press
water button to activate water; press air button to activate air. Pressing both buttons will create a mist.
WARNING: Refer to Forest Dental Unit Water Line Maintenance Operator’s Guide for detailed procedures on maintaning
syringe water line.
Air
Water
Pressing
both = mist
Air/Water Syringe
Operation
WARNING: Forest syringes are provided unsterile. Sterilization of syringe tip is required prior to use.
Syringe Infection Control
Other Forest Dental Dental Equipment manuals
Popular Dental Equipment manuals by other brands

Waterpik
Waterpik ION Water Flosser quick start guide

3M Unitek
3M Unitek Ortholux Technique guide
DENTSPLY
DENTSPLY SmartLite Focus Instructions for use

Owandy Radiology
Owandy Radiology i-max touch 3D user manual

Aseptico
Aseptico AEU-525 Transport III Operation & maintenance manual

Dentsply Sirona
Dentsply Sirona Cavitron 300 Series Instructions for use

POSDION
POSDION REXTAR X Operation & service manual

Pasta Del Capitano
Pasta Del Capitano INN 912 user manual

RAMVAC
RAMVAC BISON Service instruction

Bien-Air Dental
Bien-Air Dental Chiropro L After Sales Checking Procedure
DKL
DKL L2-S300 operating instructions

KaVo
KaVo QUATTROcare PLUS 2124 A Instructions for use