Certified Company ISO 13485 : 2016, NF EN ISO 13485 : 2016 by
LNE GMED
S
UMMAR
Y
1.
I
N
T
R
O
DUC
TIO
N ___________________________________________________________________ 5
2. LIST OF MARKING SYMBOLS _________________________________________________________ 6
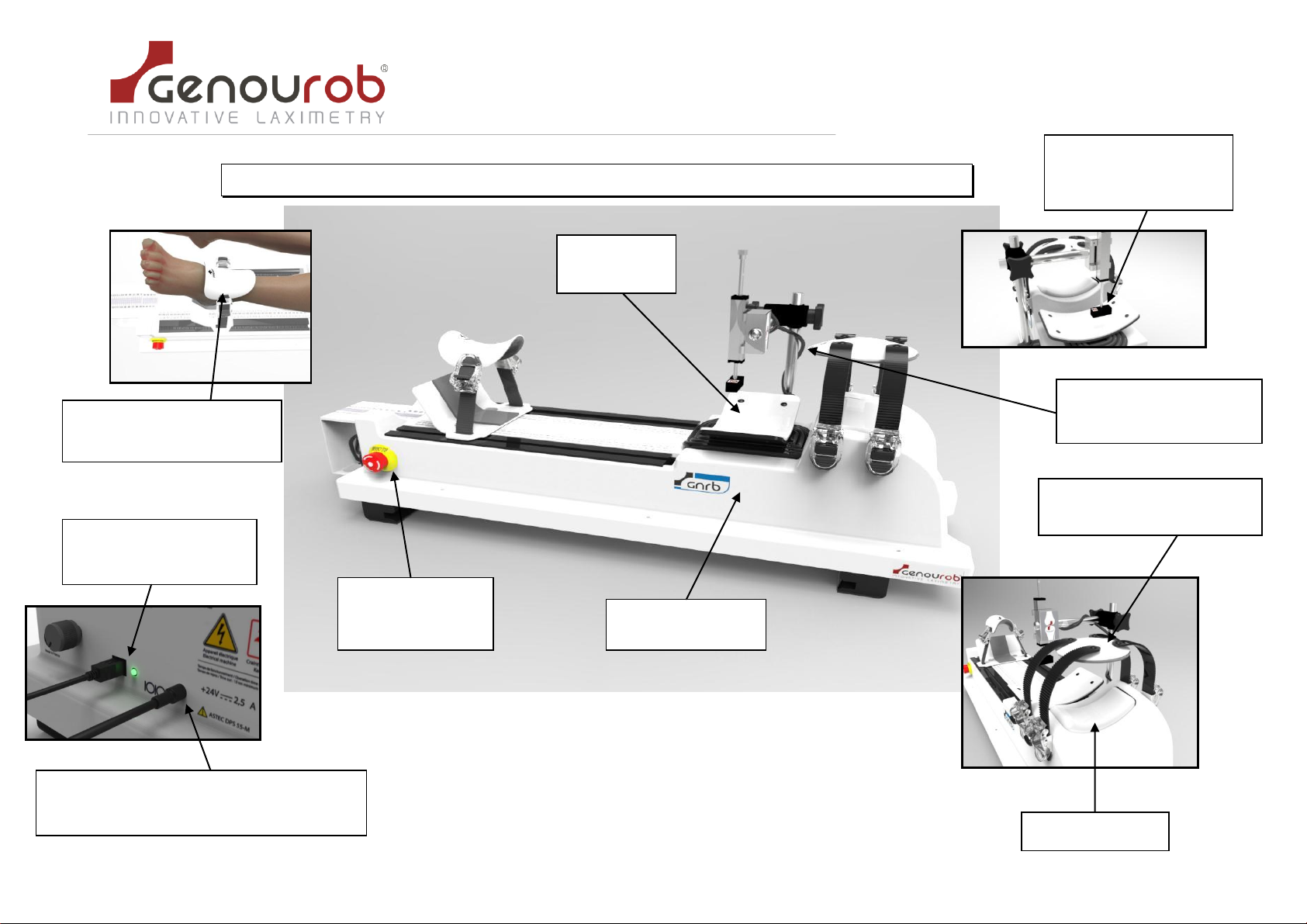
3. D
ES
CR
IPTIO
N _____________________________________________________________________ 7
4. GENERAL SAFETY INSTRUCTIONS _____________________________________________________ 8
5. INSTALLATION
G
U
I
D
E
_______________________________________________________________ 9
5.1.Installation of the GNRB® device__________________________________________________________ 9
5.1.1. Unpacking ________________________________________________________________________________ 9
5.1.2. Installation of the GNRB®__________________________________________________________________ 9
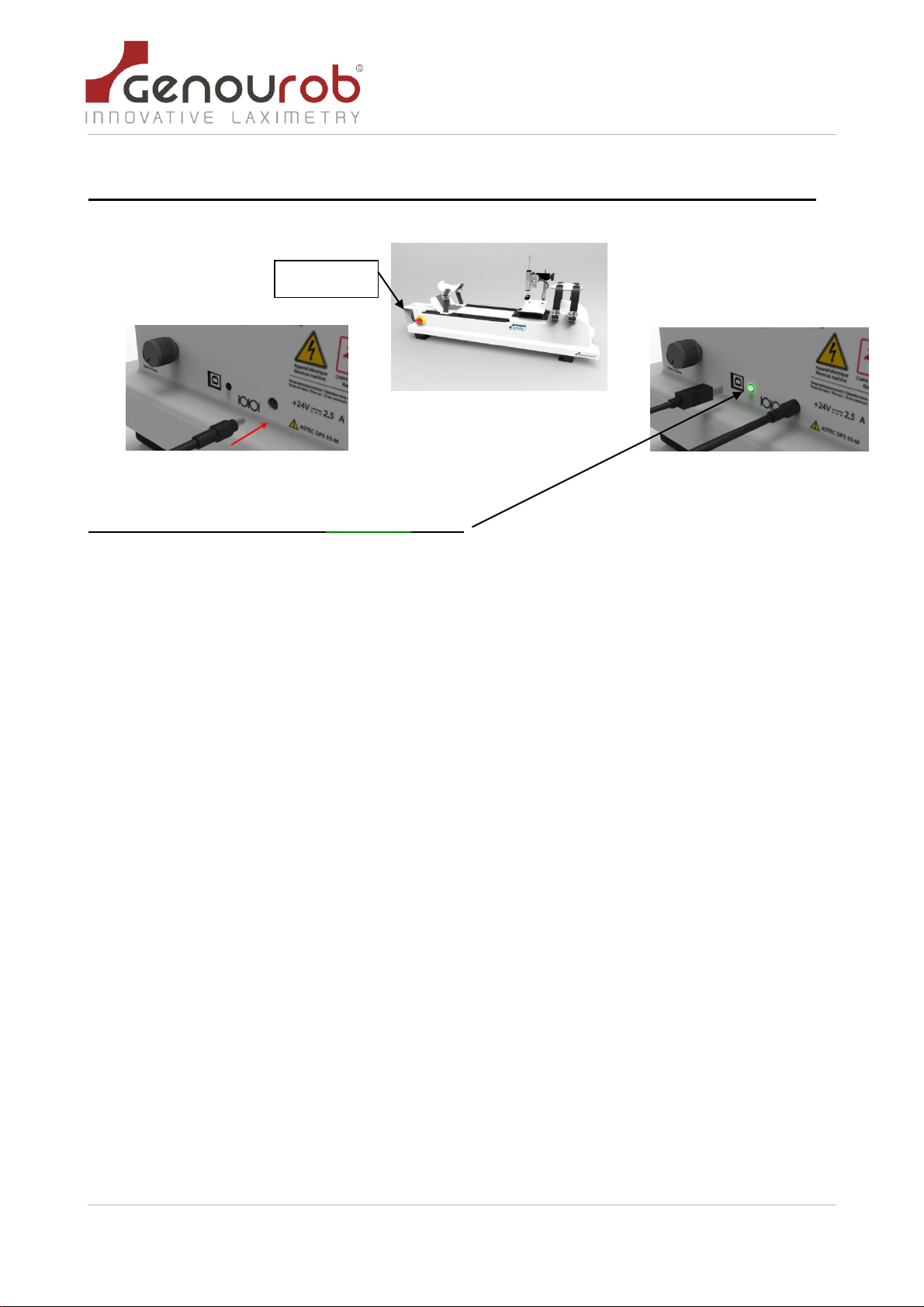
5.2.Connections _________________________________________________________________________ 10
6. USING THE GNRB® FOR THE FIRST
TI
M
E
______________________________________________ 11
6.1.Login/Password ______________________________________________________________________ 11
6.2. First time use________________________________________________________________________ 12
7. DESCRIPTION OF THE GNRB®
SOFTW
AR
E
______________________________________________ 13
7.1.Toolbar _____________________________________________________________________________ 13
7.2.Patient file tab _______________________________________________________________________ 13
7.2.1. Identity f
orm
_____________________________________________________________________________ 13
7.2.2. Medical
in
f
o
rmat
ion
______________________________________________________________________ 14
7.2.3. Additional examinations __________________________________________________________________ 15
7.3. GNRB test preparation tab _____________________________________________________________ 16
7.3.1.
S
ett
ing
s_________________________________________________________________________________ 16
7.3.2. Co
n
tr
ols
_________________________________________________________________________________ 16
7.3.3. GNRB status_____________________________________________________________________________ 17
7.3.4. Push force _______________________________________________________________________________ 17
7.3.5. Number of test measurements ____________________________________________________________ 18
7.3.6 Radiology Option ____________________________________________________________________________ 18
7.3.7. Contraction Option __________________________________________________________________________ 18
7.3.8. Data table _________________________________________________________________________________ 18
7.4.The results tab _______________________________________________________________________ 19
7.4.1. Selecting results_____________________________________________________________________________ 19
7.4.2. Graphs ____________________________________________________________________________________ 20
7.4.3. Differential Results __________________________________________________________________________ 21
7.5.Configuration tab _____________________________________________________________________ 22
7.5.1. Configuration_______________________________________________________________________________ 22
7.5.2 Print document in the PDF format_______________________________________________________________ 22
7.5.3. Information ________________________________________________________________________________ 24