Content
1Intended Use, Indications and Operation .......................................................5
1.1 Intended Use..........................................................................................5
1.2 Indications..............................................................................................5
1.3 Mode of Operation.................................................................................6
2Regulatory Compliance and Labeling..............................................................7
2.1 Regulatory Compliance...........................................................................7
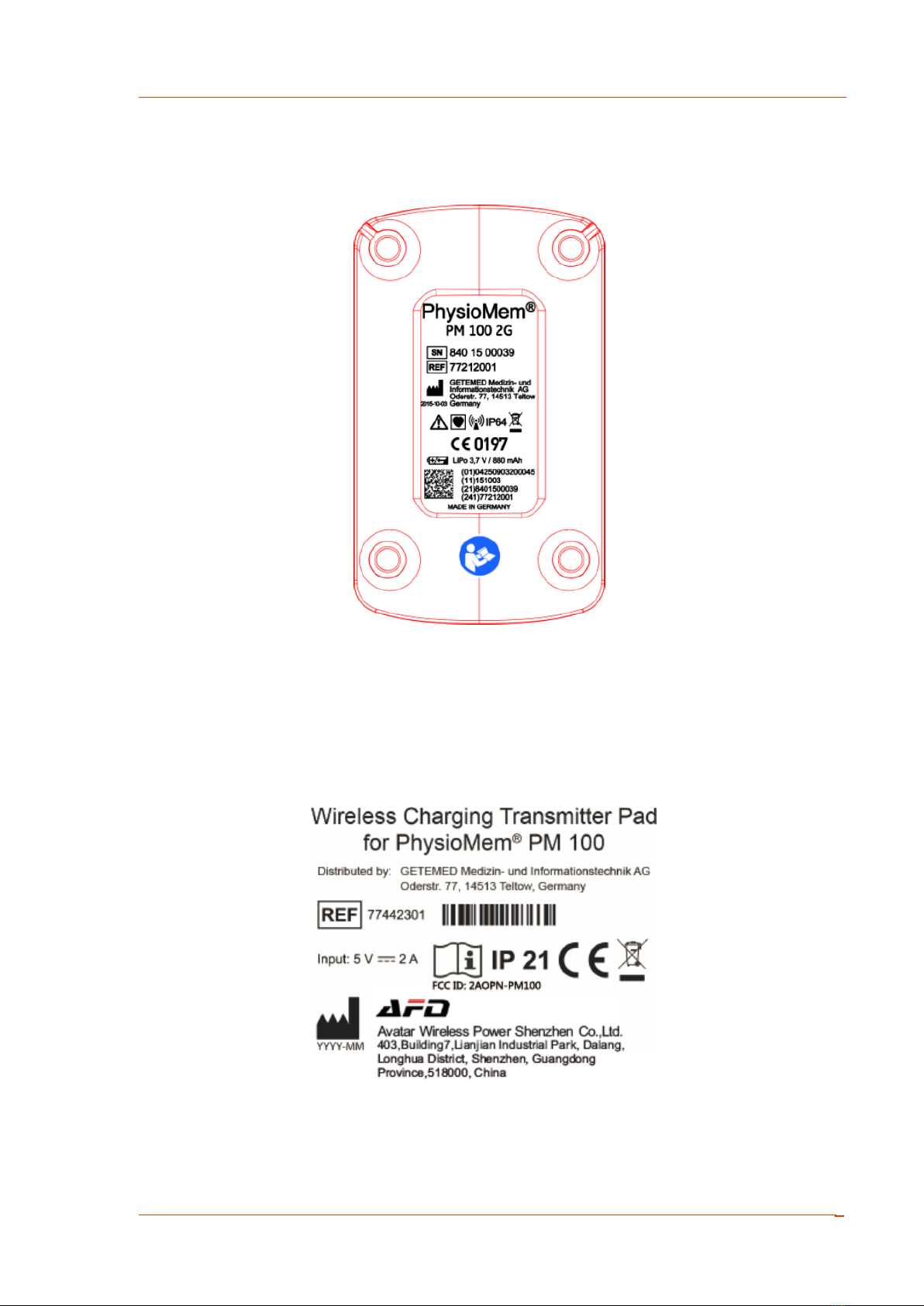
2.2 Information on the Device Labels...........................................................9
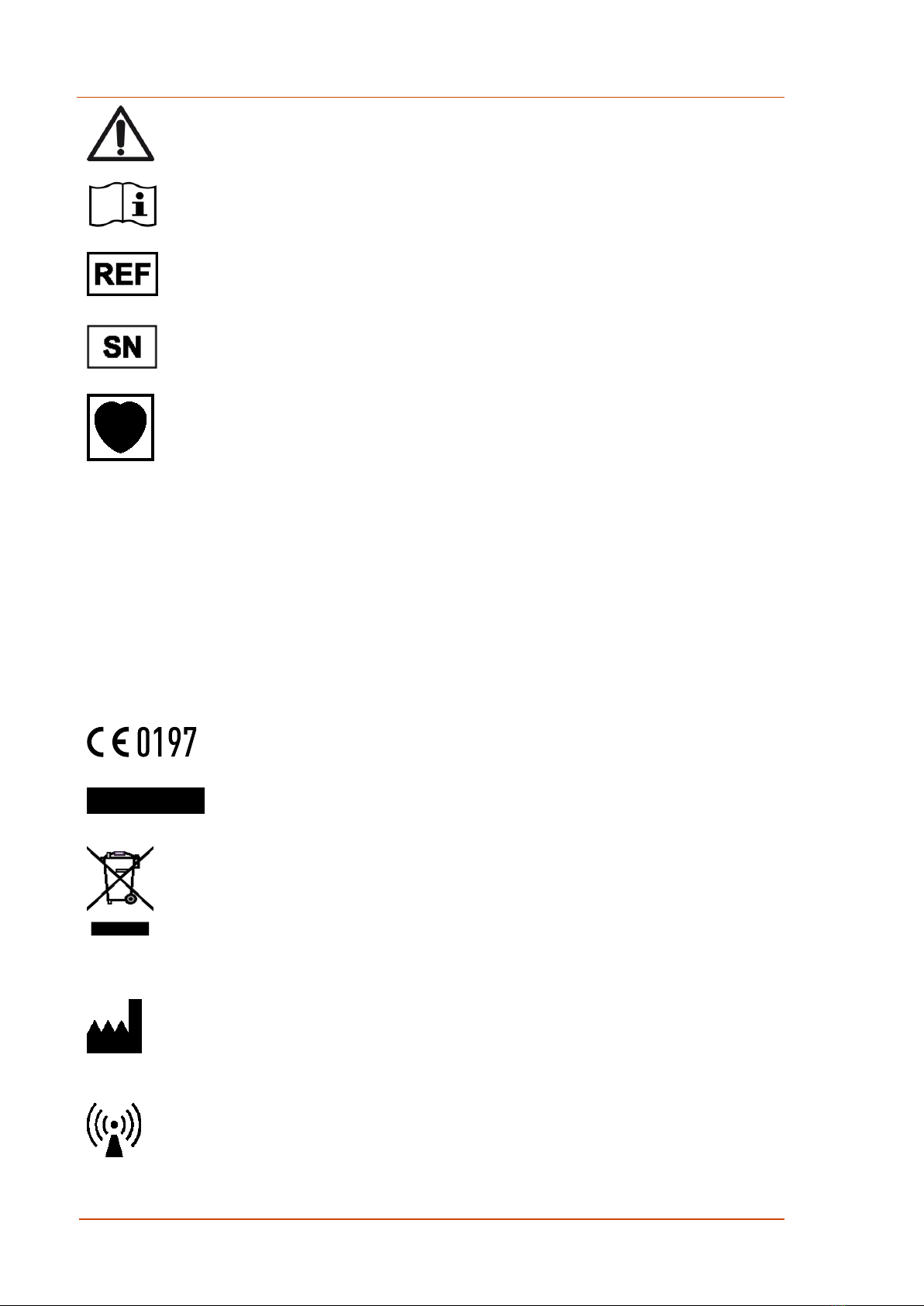
2.3 Symbols on the Packaging Label ...........................................................11
3Safety and Reliability ....................................................................................12
3.1 Definitions............................................................................................12
3.2 General Warnings.................................................................................13
3.3 General Cautions..................................................................................14
3.4 Safety and Reliability Only with Proper Maintenance...........................16
3.5 Cleaning the Recorder and Accessories ................................................17
3.6 Disposing of the Device, Batteries, and Accessories .............................18
3.7 Established Medical Practices...............................................................18
3.8 Manufacturer Responsibility.................................................................18
4Control Elements, Putting into Operation.....................................................19
4.1 Control Elements..................................................................................19
4.2 Putting into Operation, Fully Charging the Battery ...............................20
5Recording and sending an ECG .....................................................................25
5.1 How and where you apply the device...................................................25
5.2 Recording an ECG .................................................................................26
5.3 Sending an ECG Recording....................................................................28
5.4 Switching off the Device .......................................................................30
6The System of PhysioMem and ReSTA..........................................................31
6.1 Overview ..............................................................................................31
6.2 ECG Report...........................................................................................32
7Meaning of Display Symbols and Audible Notifications ................................35
7.1 Display Symbols....................................................................................35
7.2 Audible Notifications ............................................................................37