Globus Elite User manual

Rev.02.15

2
Rev.10.15
3
Rev.10.15
DEAR CUSTOMER
THANK YOU FOR CHOOSING A GLOBUS PRODUCT. WE REMAIN AT YOUR
ENTIRE DISPOSAL FOR ANY ASSISTENCE OR ADVICE YOU MAY NEED
The electrostimulators A1R (Elite) are manufactured by:
SAVIA L.t.d. - 4nd Industrial District, Feng Huang
and distributed by:
DOMINO s.r.l.
via Vittorio Veneto 52
31013 - Codognè - TV - Italy
Tel. (+39) 0438.7933
Fax. (+39) 0438.793363
E-Mail: info@globuscorporation.com
www.globuscorporation.com
This product has been manufactured according to the technical regulations in
force and is certified according to Directive 93/42/EEC updated by 2007/47
directive for medical devices, by Notified Body n. 0197.
ELITE

4
Rev.10.15
Table of contents
Device ............................................................................................................................................................ 6
Conditions of use......................................................................................................................................... 6
Technical features of the currents.......................................................................................................... 6
EQUIPMENT.......................................................................................................................................................... 7
INTENDED USE...................................................................................................................................................... 8
CONNECTIONS ...................................................................................................................................................... 9
How to connect the cables ..................................................................................................................... 9
Electrode application ................................................................................................................................ 9
Battery: how to charge the batteries.................................................................................................. 10
LABELLING AND SYMBOLS..................................................................................................................................11
Device .......................................................................................................................................................... 12
PANEL AND KEYBOARD....................................................................................................................................... 14
Display and interface............................................................................................................................... 15
ALARMS .............................................................................................................................................................. 15
Compliance................................................................................................................................................ 15
WARNINGS AND CONTRAINDICATIONS ............................................................................................................. 16
Mandatory behavior ................................................................................................................................ 16
Warnings before use................................................................................................................................. 16
Warnings during use ................................................................................................................................. 17
Side effects.................................................................................................................................................. 18
Contraindications...................................................................................................................................... 18
MAINTENANCE AND CLEANING......................................................................................................................... 19
Device .......................................................................................................................................................... 19
Battery .......................................................................................................................................................... 19
Accessories ................................................................................................................................................. 20
Disposal of the device ............................................................................................................................. 20
INSTRUCTIONS FOR USE ..................................................................................................................................... 21

5
Rev.10.15
“Program List” menu................................................................................................................................. 21
“Last 10” menu........................................................................................................................................... 23
ACTION PRINCIPLES ............................................................................................................................................ 25
Muscular electrostimulation ................................................................................................................... 25
Stimulation intensity .................................................................................................................................. 26
Tens........................................................................................................................................................ 27
PROGRAM LIST................................................................................................................................................ 29
WARRANTY ......................................................................................................................................................... 37

6
Rev.10.15
TECHNICAL FEATURES
Device
Size: 160x99x35.4 mm
Weight: 404 g
Case: in Food Grade ABS
Protection level: IP 22
Storage and transportation temperature: from -10°C to 45°C
Max. relative humidity: 30% - 75%
The values indicate the allowable limits if the product or its accessories are not in the
original package.
Conditions of use
Temperature: from 0°C to 35°C
Max. relative humidity: from 15% to 93%
Atmospheric pressure: from 700 hPa to 1060 hPa
Technical features of the currents
EMS and TENS:
compensated
Working frequency: 0.3-150 Hz
Recovery frequency: 0.3-150 Hz
Pulse amplitude: 50-450 µs
Working time: from 1 to 30 seconds
Recovery time: from 0 to 1 minute
Frequency mod. range: continuous variation from 1 to 150 Hz
Min. modulation time: 3 seconds
Amplitude modulation range: continuous variation from 50 to
450 µseconds
Charger
Brand: FLO
model: DKT-088-0200-EU
Input: 100-240V~ 50-60Hz 0, 2A
Output: 8,8 Vd.c. 0.2A
Polarity:
Channels available: Channels 1-2-3-4
Constant current: Yes
Intensity: 0-100 mA with 1000 Ohm load
Wave form: Rectangular, biphasic, symmetrical,
7
Rev.10.15
Battery
Battery pack: Ni-MH 7,2 V 1,8 Ah
EQUIPMENT
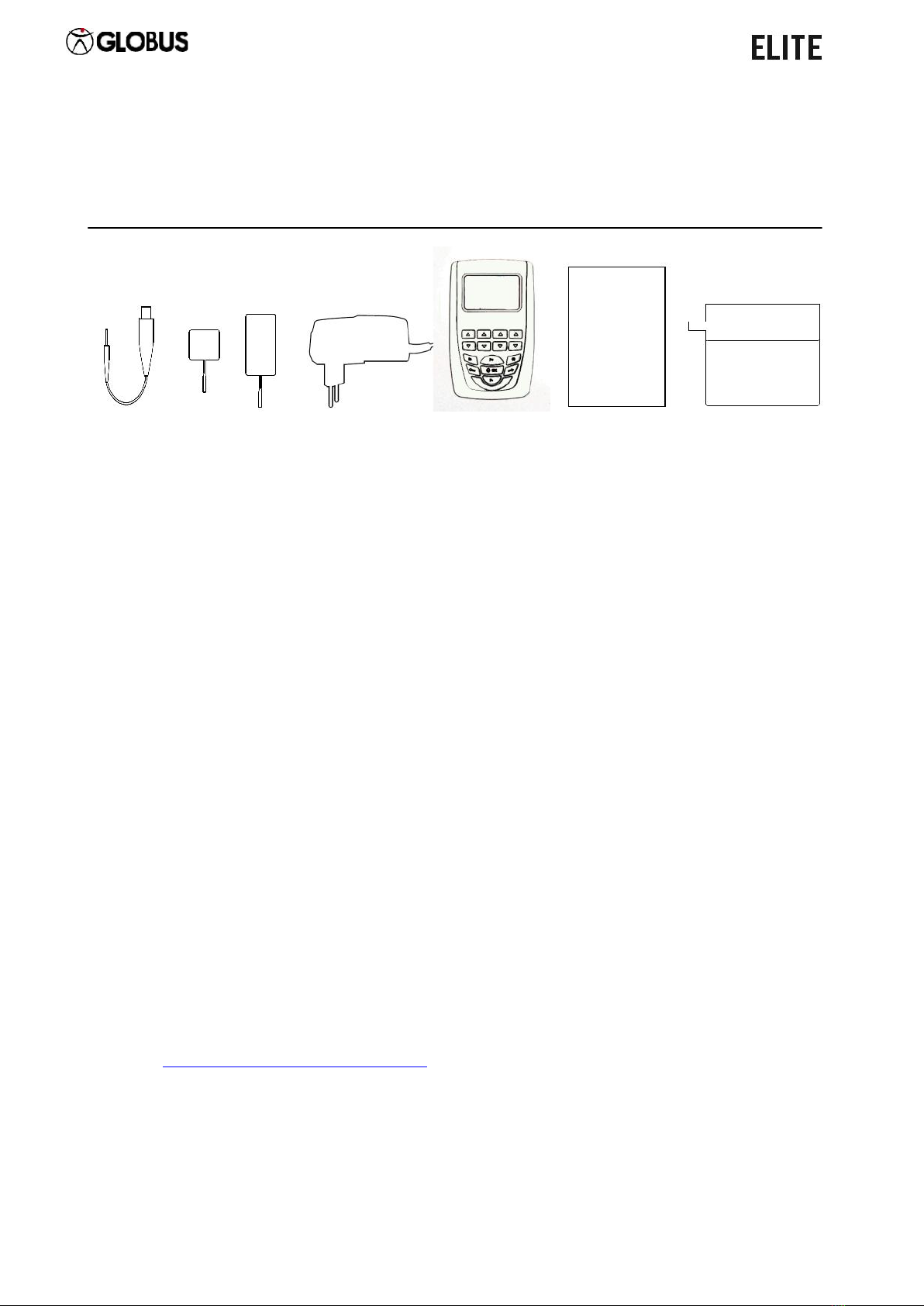
The electrostimulator is supplied complete of cables and electrodes: therefore,
please check that the package contains the complete equipment. If some
elements are not contained in the package, please contact immediately the
authorized retailer where you purchased the product.
Control the integrity of the device and its electrodes carefully.
A. 4 colored electrode connection cables (for EMS and TENS treatments)
B. A bag containing 4 reusable self-adhesive electrodes (50 x 50 mm)
(for small areas such as upper limbs, calves, cervical area…)
C. A bag containing 4 reusable self-adhesive electrodes (50 x 90 mm)
(for large areas such as thighs, abdomen and gluteal muscles...)
D. Charger (See technical features)
E. A1R Unit
F. User manual
Warranty
G. Carrying bag
All the supplied information can be modified without prior notice.
The device can be used with some optional accessories (for further info, visit the
website www.globuscorporation.com ).
If you are interested in buying these accessories, please contact the retailer.
Accessories not included (available on charge)
- Motor point pen
- Kit of 8 elastic bands for legs and thighs
A B-C D E F G

8
Rev.10.15
- Kit of 4 elastic bands for thighs
- Face electrodes
- Kit Y cables
INTENDED USE
The service life of the product is estimated at 5 years. It is advisable to return the
product for maintenance and security checks every two years. The number of
treatments depends on the battery charge. The duration of the battery is 6 months;
thereafter its replacement is recommended.
The electrostimulators are intended for use in the following operating environments:
- domestic environment;
- clinics;
- physiotherapy centers;
- rehabilitation centers;
- general pain treatments;
- beauty and sport purposes;
The device can be used by patients (appropriately informed about the conditions
of use) and the medical staff only.
9
Rev.10.15
CONNECTIONS
Cable connection outlets and power supply
Attention:
If the package, the cable or the connector of the charger show signs of wear or
damage, replace them instantly.
How to connect the cables
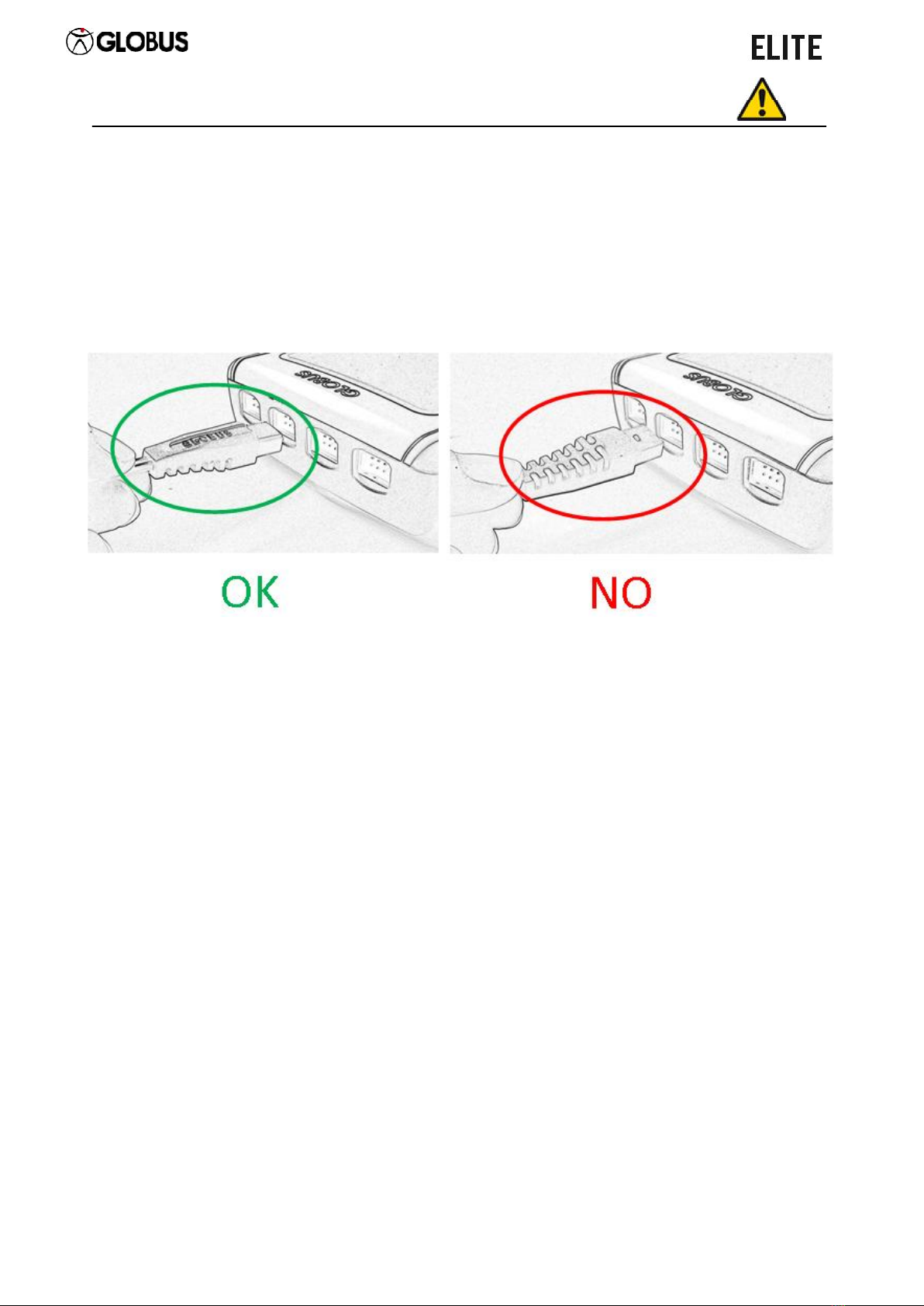
Plug the connectors in the slots in the upper part of the unit to connect the diffusers
to the device (see pic.). When plugging in the cable, the grooves of the cable have
to be oriented downwards. The inlets are placed exactly under the corresponding
channels.
ATTENTION: the images are for illustrative purposes only to show the correct insertion
of the connectors into the appropriate slots. It is recommended to unplug the
connectors grabbing the final part with the grooves and not pulling them taking the
cable in the middle.
NOTE: For EMS and TENS currents, the 4 channels with colored cables can be used
indifferently.
Electrode application
Remove the electrodes from the original package; all new electrodes have a seal
on the package. Ensure that the device is off. First, connect the two cable plugs to
the electrodes, then disconnect the electrodes from their position and apply them
on the skin. See the pictures included in this manual to place the electrodes
correctly.
After use, place the electrodes in their original position again.

10
Rev.10.15
ATTENTION: Do not unplug the electrodes if the unit is working.
Battery: how to charge the batteries
The device is supplied with a set of rechargeable nickel-metal hydrate batteries
(7.2V, 1.5Ah).
Recharge the batteries when the battery indicator on the display indicates ¼.
To charge the batteries, turn off the electrostimulator and disconnect the
electrodes, then connect the electrostimulator to the charger provided by plugging
it in the appropriate inlet (see picture above).
Use the charger contained in the package only. Contact the authorized service
center to replace the batteries.
Table of contents
Other Globus Personal Care Product manuals