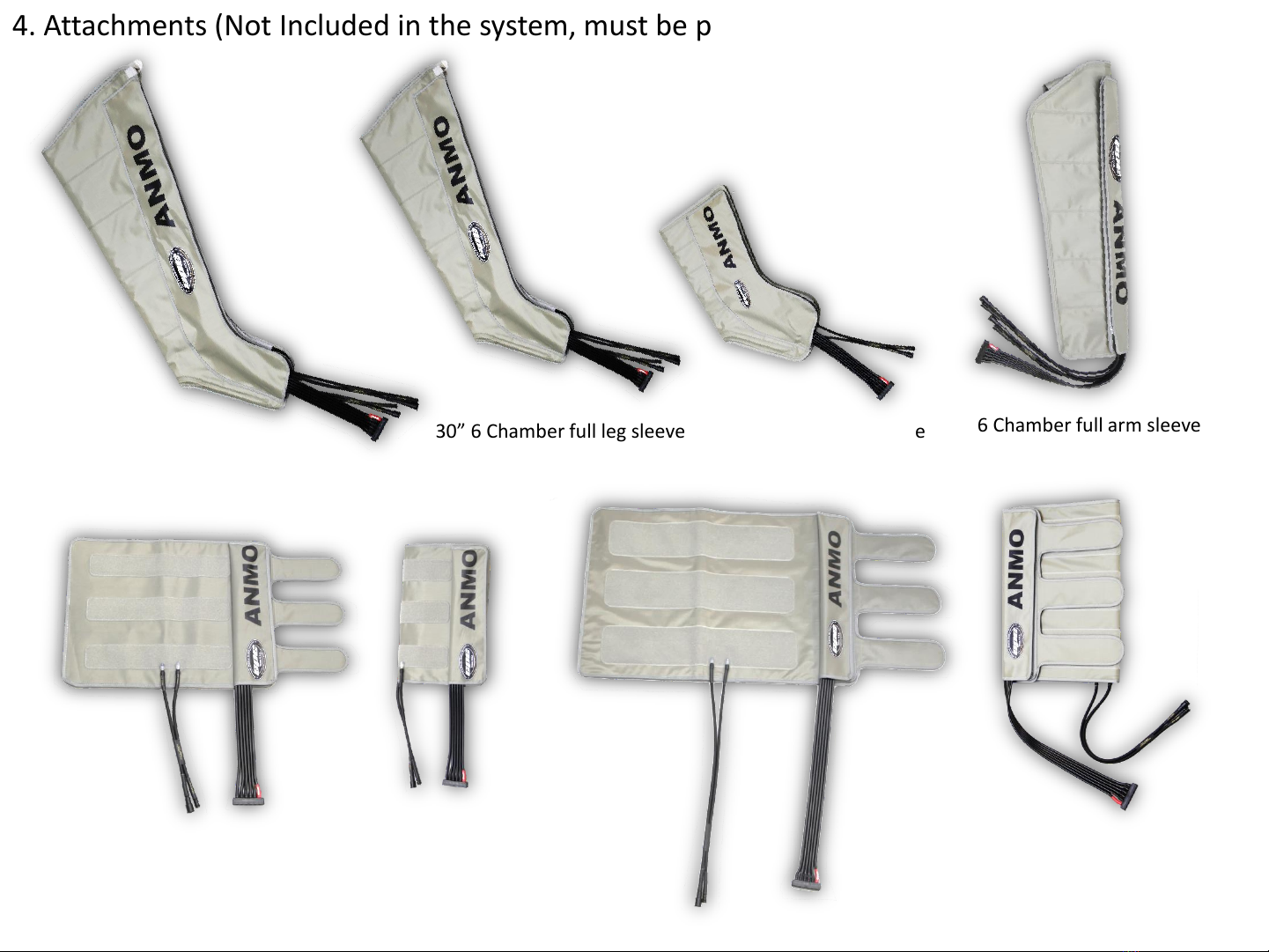
Thank you for choosing ANMO. To assure you have the best experience possible, please
be sure to read the entire Instruction Manual prior to using the product.
1. Safety Information
• Please do not dismantle any part of the system. Please contact the vendor if any repairs are required.
• In order to prevent damage to the device, please do not twist or bend the water lines.
• Avoid puncturing the sleeves and tubing. If any defects or blemishes are found in the sleeves and tubing, please cease use.
• ANMO could be cold enough to seriously damage skin.
• ANMO could reach pressure high enough to damage muscle and blood vessels. Please use the device with caution.
• Many device functions can be manually controlled; the user must stay alert and sober while operating.
• The air pump system is semi-manual; amount of pressure and options to inflate or deflate are mainly controlled by the user.
For the sake of safety, please cease use of the device in the event of numbing or lack of sensation in the affected limbs.
• Wrap deflation: Turning off the system will automatically open the air-release valve and release air from the wrap.
• Improper placement or prolonged use of ANMO could result in tissue damage from frostbite or inhibited blood circulation.
Please be attentive while using this device.
• During ANMO treatment, patients should monitor the skin surrounding the treated region or the digits of the extremities of the
treated limb for any burning, itching, increased swelling, or pain. If any of these signs are present, or any changes in skin
appearance occur (such as blisters, increased redness, discoloration, or other noticeable skin changes), patients
are advised to discontinue use and consult a physician.
• ANMO sleeves are not sterile; do not place directly against open wounds, sores, rashes, infections, or stitches. May be applied
over clothing or dressing.
• ANMO sleeves are available in multiple configurations but are not intended for all possible orthopedic uses. Use caution when
selecting a particular wrap for use on anatomic regions other than that for which the wrap is indicated and closely supervise
the user’s status. For example, the arm wrap is not designed for use on the legs.
• ANMO should be used only under the supervision of a licensed healthcare practitioner in users:
- Who have a wound in the affected region (the wound must be dressed prior to use of ANMO).
- Who have an acute, unstable (untreated) fracture in the affected region.
- Who are children under 18 years old or patients who have cognitive disabilities or communication barriers, whether
temporary due to medication or permanent.
- Who have a cardiac insufficiency or congestive heart failure (with associated edema in the extremities or lungs).
- Who have a localized skin condition (e.g., dermatitis, vein ligation, gangrene, skin graft) in the affected region.
- Who have erysipelas or other active infections in the affected region.
WARNINGS
Page 2