Hanna Instruments GroLine HI98168 User manual
Other Hanna Instruments Measuring Instrument manuals

Hanna Instruments
Hanna Instruments HI97769 User manual

Hanna Instruments
Hanna Instruments HI96800 User manual

Hanna Instruments
Hanna Instruments HI 140 Series User manual

Hanna Instruments
Hanna Instruments HI 93114 User manual
Hanna Instruments
Hanna Instruments HI 99163 User manual

Hanna Instruments
Hanna Instruments HI 991001 User manual

Hanna Instruments
Hanna Instruments HI 95724C User manual

Hanna Instruments
Hanna Instruments HI 9142 User manual

Hanna Instruments
Hanna Instruments HI 99181 User manual

Hanna Instruments
Hanna Instruments 96708 User manual
Hanna Instruments
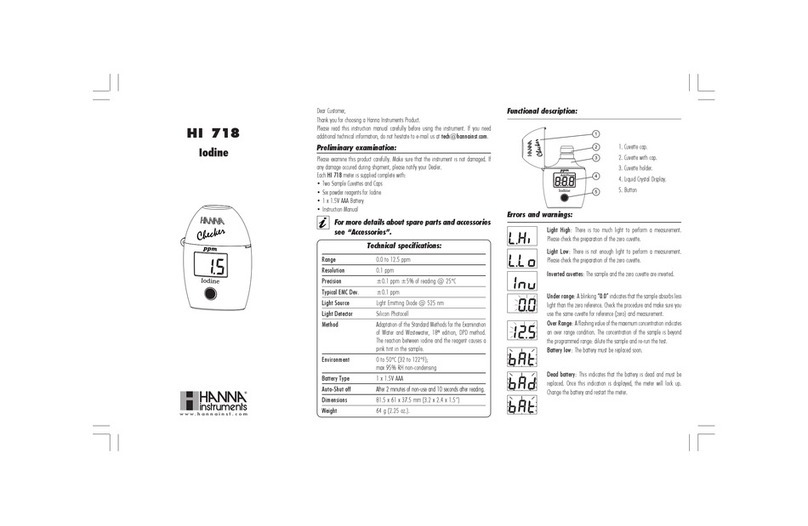
Hanna Instruments HI 718 User manual

Hanna Instruments
Hanna Instruments GROLINE MONITOR HI981420 User manual

Hanna Instruments
Hanna Instruments HI97720 User manual

Hanna Instruments
Hanna Instruments HI98192 User manual

Hanna Instruments
Hanna Instruments HI 717 User manual

Hanna Instruments
Hanna Instruments HI9813-51 User manual

Hanna Instruments
Hanna Instruments HI 93703 User manual

Hanna Instruments
Hanna Instruments HI 761 User manual

Hanna Instruments
Hanna Instruments HI 771 User manual

Hanna Instruments
Hanna Instruments HI747 User manual
Popular Measuring Instrument manuals by other brands

Powerfix Profi
Powerfix Profi 278296 Operation and safety notes

Test Equipment Depot
Test Equipment Depot GVT-427B user manual

Fieldpiece
Fieldpiece ACH Operator's manual

FLYSURFER
FLYSURFER VIRON3 user manual

GMW
GMW TG uni 1 operating manual

Downeaster
Downeaster Wind & Weather Medallion Series instruction manual

Nokeval
Nokeval KMR260 quick guide

HOKUYO AUTOMATIC
HOKUYO AUTOMATIC UBG-05LN instruction manual

Fluke
Fluke 96000 Series Operator's manual

Test Products International
Test Products International SP565 user manual

General Sleep
General Sleep Zmachine Insight+ DT-200 Service manual

Sensa Core
Sensa Core Lacto Spark user manual