HEMOSTATIX PRECISION 8400 User manual
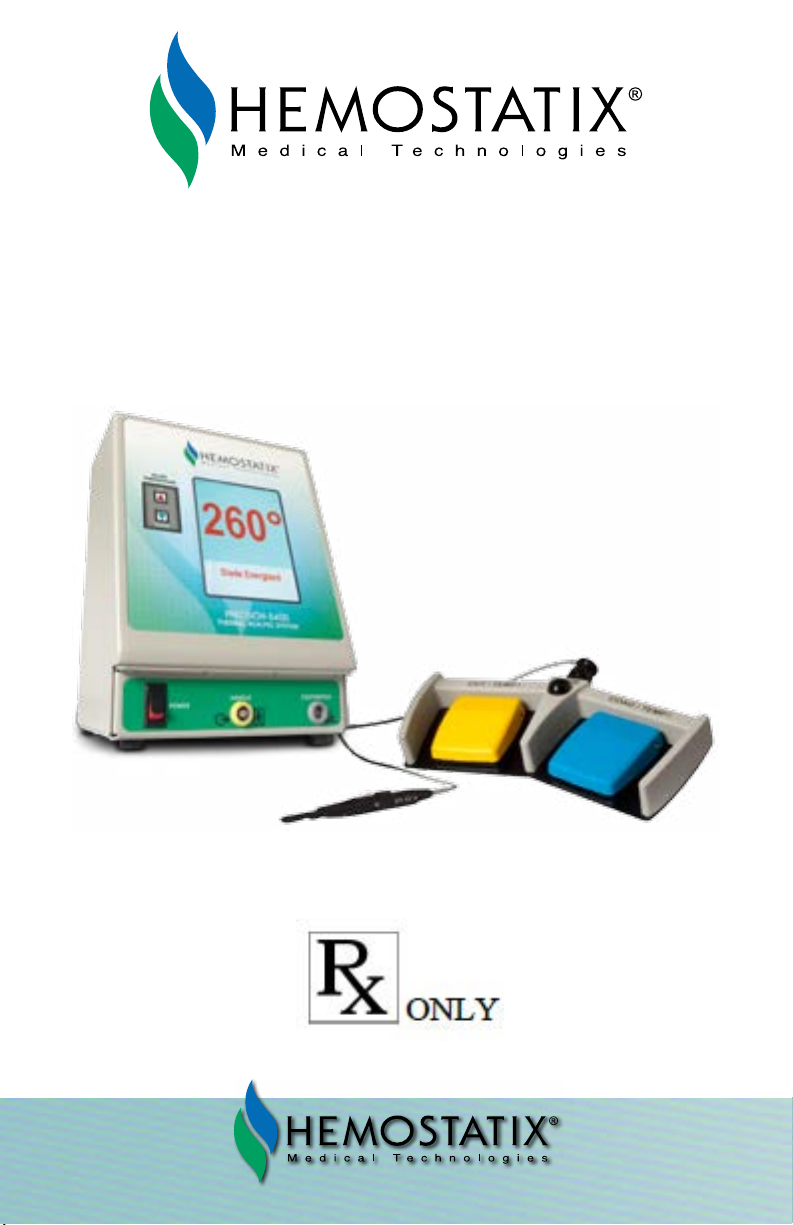
OPERATING MANUAL
THERMAL SCALPEL SYSTEM
MODEL PRECISION 8400 CONTROLLER
Part Number 7013-8400
For use with the Model 9050 Disposable Scalpel Handles

2 3
CONTRAINDICATIONS
The Hemostatix Thermal Scalpel System is contraindicated in the
presence of a FLAMMABLE ANESTHETIC MIXTURE WITH AIR
or OXYGEN or NITROUS OXIDE.
POTENTIAL ADVERSE EFFECTS
Known potential adverse effects include, but are not limited to,
thermal injury to tissue, including nerves or other delicate tissues, and
inability to effectively provide hemostasis of larger vessels.
SYSTEM WARNINGS
1. EXPLOSION HAZARD – The Hemostatix Thermal Scalpel
System is contraindicated in the presence of a FLAMMABLE
ANESTHETIC MIXTURE WITH AIR or OXYGEN or
NITROUS OXIDE.
2. NO MODIFICATION OF THIS EQUIPMENT IS ALLOWED.
3. Electrical shock hazard. Do not remove cover. Refer to
manufacturer for service.
4. Do not attach unapproved components to the HTSS unit to avoid
electrical shock.
5. Ensure the sound volume is adequately adjusted to that alarms
and alerts are clearly heard
6. To avoid risk of electric shock, this equipment must only be
connected to a supply mains with protective earth.
7. Do not place the HTSS controller unit in direct contact with or
within 1 m of any type of electro-surgical equipment. This
equipment has been tested and found to comply with the limits for
medical devices to the IEC 60601-1-2:2014.These limits are
designed to provide reasonable protection against harmful
interference in a typical medical installation. This equipment
radiates electromagnetic fields and, if not installed and used in
accordance with the instructions, may cause harmful interference.
However, there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful
interference to other devices, which can be determined by turning
the equipment off and on, or is affected by interference
INTRODUCTION
HEMOSTATIX THERMAL SCALPEL BLADES are similar in size,
shape, and sharpness to traditional steel scalpel blades; however,
Hemostatix blades can be heated to a user-selected temperature
appropriate for sealing small vessels as they are cut.
To cut and simultaneously seal blood vessels effectively with minimum
tissue damage, the sharp steel cutting edge of a heated scalpel blade
must be uniformly maintained at the desired temperature within
narrow limits. The Hemostatix Thermal Scalpel System utilizes micro-
circuitry incorporated within the blade itself to maintain the cutting
edge temperature within the necessary tolerance, selectively delivering
additional thermal energy only to those regions of the blade using heat
due to tissue contact. By so doing, the Hemostatix Thermal Scalpel
System automatically compensates for the varying degrees of heat loss
that occur during surgical procedures (depending on the type of tissue
being incised and the rate at which cutting is carried out), maintaining
the cutting edge in the desired temperature range.
In contrast with electrosurgical devices, the Hemostatix Thermal Scalpel
System passes no electrical current through the patient, and there is no
sparking or electrical arcing to the tissue. Electrosurgical devices “cut”
and/or cauterize using electrical currents which pass through the patient
vaporizing tissue at the point of contact and generating heat and tissue
damage down the path of the electrical current. The Hemostatix
Thermal Scalpel System cuts tissue with a sharpened steel edge, like a
conventional cold-steel scalpel blade, and simultaneously seals blood
vessels using heat thermally conducted to the tissue from an elevated-
temperature blade which is electrically insulated from the patient. By
thermally transferring heat from
a uniformly- controlled, essentially constant temperature blade,
the amount of tissue damage associated with hemostatic cutting is
minimized.
INDICATIONS FOR USE
The Hemostatix Thermal Scalpel System is a surgical instrument
designed to retain the precise, clean cutting characteristics of
the traditional steel scalpel while minimizing blood loss by
simultaneously sealing blood vessels as they are cut, with minimum
damage to surrounding tissue and virtually no muscle stimulation, using
heat thermally conducted to the tissue from an elevated-temperature
blade.
Rx Only – CAUTION: Federal (U.S.A.) law restricts this device to
sale by or on the order of a physician.
NOTICE TO USERS AND/OR PATIENTS
Any serious incident that has occurred in relation to the device should
be reported to the manufacturer and the competent authority of the
Member State in which the user and/or patient is established.

4 5
from other devices, the user is encouraged to try to correct the
interference by one or more of the following measures:
•Reorient or relocate the receiving device.
•Increase the separation between the equipment.
•Connect the equipment into an outlet on a circuit dierent
from that to which the other device(s) are connected.
•Consult the manufacturer.
8.The HTSS’s maintenance port is to be used by Hemostatix
personnel only. The port is not to be accessed, for any purpose, by
the customer. Any attempt to connect via the maintenance port
will result in a termination of any warranties that may exist and
may damage the unit.
9.The HTSS’s auxiliary port is not to be utilized for any other
purpose other than to power equipment specifically designed
by Hemostatix. Any attempt to plug, non Hemostatix equipment
into the output connector may result in damage to the
Hemostatix unit thereby terminating any warranties and may
result in an unsafe electrical condition, increased electrical
emissions, or decreased immunity of the Hemostatix system.
Anyone who connects additional equipment via the auxiliary
output terminal is therefore responsible for configuring a medical
system and is responsible that the newly configured system
complies with the requirements of the system standards IEC
60601-1 and IEC 60601-1-2. If the user has questions regarding
any connections to the AUX output port, they should contact
Hemostatix Medical Technologies.
SYSTEM PRECAUTIONS
1. It is important that the Hemostatix ermal Scalpel System
(HTSS) operator be familiar with the System’s Operator’s
Manual, its precautions, procedures, and safety issues. Read the
complete operators manual before using this equipment.
2. Do not position the HTSS unit to make it dicult to remove and
insert the unit’s separable power cord plug.
3. Hazardous electrical and thermal outputs. is equipment to be
used only by qualied medical personnel.
4. Disconnect power to the HTSS before cleaning the unit to avoid
electrical shock.
5. To avoid the risk of electrical shock, achieve electrical grounding
reliability with proper connections. Connect the HTSS unit to
hospital grade receptacles only.
6. e HTSS should not be used adjacent to or stacked with
other equipment. If adjacent or stacked use is necessary, the
HTSS should be observed to verify normal operation in the
conguration in which it will be used.
7.
8
Do not operate the HTSS in the presence of Magnetic Resonance
Imaging Equipment.
The recommended temperature setting for skin incisions is 70°
C. For minimal scarring, make the initial skin incision with
the scalpel handle in the OFF position. Cutting with the blade
unheated will eliminate the possibility of superficial skin scars due
to contact with a heated blade.
9.User should select the lowest set point temperature that will afford
adequate hemostasis for the maximum anticipated rate of tissue
cutting, thereby minimizing unnecessary (thermal) necrosis of
tissue.
10.Care should be taken when using the HTSS to dissect around
nerves and other delicate structures to avoid thermal injury to
these structures.
11.The Model P8400 Hemostatix Thermal Scalpel System needs
special precautions regarding EMC and needs to be installed and
operated according to the information in the tables presented at
the back of this manual and portable and RF communications
equipment can affect the operation of the product.
12.All service must be performed by Hemostatix Medical Technology
personnel only.
13.Repair and/or modification the HTSS by anyone other than
qualified service personnel may significantly compromise the unit’s
ability to perform effectively and/or void the equipment warranty.
14.Opening the HTSS unit and/or breaking the tamper-proof seal
will void the equipment warranty.
15.Hemostatix Thermal Scalpel handles are single-use devices.
If required by local code, connect the HTSS unit to the hospital
equalization connector with an equipotential cable.
Lists of all compatible components of the Model P8400
Hemostatix Thermal Scalpel System are provided on pages
14-15 of this manual.
16.
17.

6 7
19.The Handle and Footswitch connector ports on the front of the
Hemostatix controller are not to be utilized for any other purpose
than to connect to specified compatible components designed by
Hemostatix.
20.DO NOT allow saline, or any other fluid, to enter the handle
during use. Saline is highly conductive and will interfere with the
circuitry inside the handle causing the handle not to work
properly.
21.Rx Only – CAUTION: Federal (U.S.A.) law restricts this device
to sale by or on the order of a physician.
22.USE WITH PLASTIC ADHESIVE DRAPES – When the skin
incisions are to be made through a plastic adhesive skin drape, use
the scalpel handle and blade in the OFF position (handle switched
OFF).
23.USE WITH ELECTROSURGICAL UNITS – DO NOT touch
the Hemostatix Thermal Scalpel blade to any electrosurgical
(e.g., Bovie) tip as significant damage to the Hemostatix Thermal
Scalpel controller unit will result. Keep at least 1 cm between the
blade and the electro surgical tip. The Hemostatix Thermal
Scalpel CANNOT BE USED to conduct electro-surgical current
through clamps.
24.AVOIDING INADVERTENT PATIENT BURNS – DO NOT
rest the Hemostatix Thermal Scalpel handle and/ or blade on
surgical drapes or on the patient during use. When energized, the
blade is sufficiently hot such that patient burns can result from
inadvertent patient contact. When the Hemostatix Thermal
Scalpel handle is not being used, it is HIGHLY
RECOMMENDED that the handle ON/OFF switch be
positioned OFF. Care should be taken to avoid unintended
activation of COAG mode by inadvertently depressing the
COAG button or foot pedal.
25.GROUNDING – Reliability can only be achieved when the
equipment is connected to a properly equivalent receptacle
marked “Hospital Grade”.
26.STERILIZATION – The Model P8400 Hemostatix Thermal
Scalpel Handle is provided STERILE provided the primary sterile
packaging is unopened and undamaged.
27.Remove and discard of used disposables following local
regulations for proper disposal of contaminated material.
28.Electrical shock hazard. Do not remove cover. Refer to
manufacturer for service.
29.If the user has any questions regarding compatibility of accessories
or cables they should contact Hemostatix Medical Technologies,
LLC.
18. In the unlikely event that the handle is unable to be turned off
normally due to a mechanical failure or due to debris in the
handle, the controller should be turned off and the disposable
replaced
COMPONENT PRECAUTIONS
1. Model P8400 Hemostatix ermal Scalpel System Blades are
provided sterile and ARE NOT intended for reuse.
2. Blades are surgically sharp and used blades may be extremely
hot to the touch. Always use a sponge, clamp or hemostat to
grasp the used blade. Always follow proper sharps precautions
when handling a blade and biohazard disposal techniques when
discarding a used blade.
3. DO NOT BEND THE BLADE – Care should be taken not to
bend the blade while cleaning, insertion, or reinsertion as the
heater leads may become broken and the blade stop working.
4. e HTSS blade’s non-stick coating cleans most eectively when
hot. Best results are obtained using dry 4x4 gauze when the blade
is hot.
5. Accurate calibration can only be achieved if the blade is at
room temperature when it is inserted into the handle. If the
blade becomes accidentally dislodged from the handle, turn the
handle OFF, dip the blade in sterile water to cool it to room

8 9
temperature, and then reinsert it.
6. Never use any type of abrasive pad to clean the blades. The
abrasives will damage the circuit and render the blade unusable.
7. To remove a blade from the handle, pull the blade straight out of
the handle. Bending, twisting or flexing the blade could damage
the blade contacts and retainers within the handle causing it to no
longer function.
8. If you are getting multiple error messages during blade insertion,
try inserting the blade into the handle BEFORE plugging the
handle into the controller unit.
9. DO NOT allow saline, or any other fluid, to enter the handle
during use. Saline is highly conductive and will interfere with the
circuitry inside the handle causing the handle not to work
properly.
10. DO NOT use any type of instrument (e.g. hemostats) to insert
the blade into the handle as this would damage the blade’s
imprinted circuitry and render it inoperable.
11. The handle must be energized for the COAG switch to work.
12. DO NOT immerse the handle in liquid of ANY KIND. The
handle contains electronic contacts and moisture sensitive
electrical components which can be damaged and fail to function
if immersed in liquids of any kind. DO NOT allow any solution
to penetrate to the interior of the handle.
13. External Cleaning is the only controller maintenance that can be
performed by the user.
14. Servicing the controller unit by other than and qualified service
personnel approved by Hemostatix Medical Technologies, LLC
renders the Warranty void.
15. Before cleaning the controller, detach the controller unit from
the AC power source.
16. DO NOT immerse the console.
17. DO NOT use an abrasive cloth or cleaners, especially on the
display screen.
18. DO NOT dispose of this product in the unsorted municipal
waste stream. Dispose of this product according to local
regulations.

10 11
e Model P8400 Hemostatix ermal Scalpel System consists of
four components:
1.
2.
CONTROLLER – An electronic power supply/controller that
energizes the blade and provides various automatic calibrations,
sensing, and control functions. It has user controls with visual and
audible indications of instrument status.
SINGLE-USE, DISPOSABLE HANDLE – A single-use,
disposable handle connected to the controller unit with a light-
weight, flexible electrical cable. Disposable scalpel blades are
inserted into the disposable handle. The user is able to control
blade temperature, on/off and COAG modes directly from the
handle.
3. DISPOSABLE BLADES – Various sizes and shapes of sterile
disposable scalpel blades are available which are similar in size and
shape to conventional cold-steel scalpel blades. Blades are single-
use only and should never be reused. e Hemostatix blades
incorporate heating and temperature-sensing micro-circuitry
which provides heat for hemostasis and sensing feedback to the
controller. DO NOT bend the blade – Care should be taken not
to bend the blade while cleaning, insertion, or reinsertion as the
heater leads may become broken and the blade stop working.
4. FOOTSWITCH – An optional footswitch (REF 7013-8410) is
available which allows the surgeon to set the desired temperatures
of the blades as well as activate CUT or COAG modes. e
footswitch has two modes: (1) CUT/COAG and (2) TEMP UP/
DOWN. Switching from mode (1) to mode (2) and vice-versa
is controlled by depressing the black MODE button on the
top of the footswitch. When in the TEMP UP/DOWN mode,
depressing and releasing the left (yellow) pedal will decrease the
set point temperature by 10° C; whereas, depressing and releasing
the right (blue) pedal will increase the set point temperature by
10° C. When in the CUT/COAG mode, depressing and holding
the left (yellow) pedal will energize the scalpel blade to come to
the selected temperature. Similarly, depressing and holding the
right (blue) pedal will energize the scalpel blade to come to the
COAG temperature of 300° C.
IMPORTANT FEATURES
SURGICAL FEATURES
1. Retains the Precision of Surgical Steel – ermal Scalpel blades
are similar in size and shape to conventional scalpel blades and
have the same sharp surgical steel cutting edges to retain the
precision and “feel” of the conventional scalpel when cutting.
2. Reduces blood Loss – e ermal Scalpel conducts heat from
SYSTEM DESCRIPTION
MODEL P8400 HEMOSTATIx
THERMAL SCALPEL SYSTEM
1
4
3
2

12 13
its sharp, heated blade to a thin layer of tissue adjacent to the
cutting edge. e heat seals most blood vessels (less than 2mm in
diameter) as they are cut, producing near- bloodless incisions with
the precision of sharp surgical steel.
3. Maintains a Clean, Dry Surgical Field – e ermal Scalpel
seals as it cuts tissue, largely eliminating the ow of blood into
the incised area. This clean, clear operative field contributes to
improved precision and visibility at the incision site.
4. Minimizes Tissue Damage – Hemostatic incisions made with
the ermal Scalpel result in visibly less tissue damage than
when electrosurgical units are used. Independent experiments
have shown that the breaking strength and infection resistance of
healing wounds made with the Hemostatix ermal Scalpel were
essentially equal to those obtained with conventional cold-steel
scalpels and better than those made with electrosurgical units.
5. Shortens Surgery – Experience indicates that a net reduction
in overall operating time generally results when an appropriate
technique is developed and with sucient experience in the use of
the ermal Scalpel.
6. Eliminates Patient Currents and Muscle Stimulation – Since
no electrical current passes through the patient when using the
Hemostatix ermal Scalpel System, a grounding pad is not
needed and the risk of accidental electrical current burns at
grounding sites is eliminated. Muscle stimulation associated with
passing electrical currents through the body is also eliminated,
improving surgical precision.
SYSTEM FEATURES
1. Sterile, Disposable Scalpel blades – Hemostatix Thermal Scalpel
blades are individually packaged sterile and ready for use. They are
discarded when they become dull, just like conventional cold-steel
scalpel blades. Blades are single-use only and should never be
reused.
2. Automatic Calibration – The Hemostatix Thermal Scalpel
System automatically calibrates each blade, typically within 6
seconds of its insertion into the handle. The blade is ready to be
energized as soon as the calibration is complete.
3. User-Selectable Cutting Temperature – The user is able to select
the desired cutting temperature over a range of 70° C to
300° C in increments of 10° degrees C using the front panel
“Temperature Up” ▲or “Temperature Down” ▼buttons OR
by using the UP/DOWN arrows on the handle. ermal COAG
Mode – e Hemostatix ermal Scalpel System provides a high
temperature Thermal COAG Mode, suitable for sealing vessels
not sealed as they are cut. The blue button on the handle
provides convenient switching between the selected cutting and
Thermal COAG temperatures. The Thermal COAG Mode is
preset to 300° C.
4. Audible Signals – e controller provides audible tones to
indicate certain system functions and status. e signal volume
can be turned up or down by the rotating switch on the back
of the unit. e audible functions include: pressing any button
on the console or handle, blade heating, blade cooling, ermal
COAG, and blade removal. Also, a tone is heard when there are
certain problems with the system. Ensure the sound volume is
adequately adjusted so that alarms are clearly heard.
5. Visual Displays – e Model P8400 controller has one display
on the front of the unit.
6. Equipotential – Uniform potential. Means for eliminating
noise or interference with sensitive equipment by application
of a potential equalization conductor. If required by local code,
connect the HTSS unit to the hospital equalization connector
with an equipotential cable.

14 15
HANDLE CONTROLS AND INDICATORS
1. ON/OFF SWITCH - Sliding the yellow ON/OFF switch rearward
(toward the cable) mechanically latches the switch and activates the
blade. Tangerine dot is visible when switch is in the “ON” position.
Sliding the switch forward (toward the blade) deactivates the blade.
2. THERMAL COAG SWITCH - Depressing the blue button on the
handle activates the Thermal COAG mode as long as the switch is
depressed. Releasing the switch causes the temperature to revert to
the original temperature setting. NOTE: COAG mode can only be
activated if the on/off switch is in the ON position.
3. BLADE - The blade/hub assembly includes a magnetic interface.
The blade/hub should automatically seat fully into the Disposable
Handle with zero force required when the blade/hub assembly is
placed into the mating portion of the Disposable Handle. NOTE:
The handle should be in the “OFF” position before inserting the
blade.
4. TEMPERATURE CONTROL SWITCHES - With the ON/OFF
switch in the “OFF” (forward) position, depressing the UP ‘▲’
arrow temperature control switch increases the temperature.
Depressing the down ‘▼’ arrow temperature control switch
decreases the temperature. The temperature changes as long as the
switch is depressed. The temperature remains at the last setting when
the switch is released.
HANDLE, BLADE, AND FOOTSWITCH COMPATIBILITY
1. HANDLE Compatibility – The Model P8400 Hemostatix Thermal
Scalpel Controller (REF 7013-8400) is compatible with the following
Hemostatix Thermal Scalpel handle/cable configurations:
•REF 7013-9050–Model P8400 Handle w/Integral Cable Assembly–Qty 1.
•REF 7023-9050–Model P8400 Handle w/Integral Cable Assembly–Qty 6.
2. BLADE Compatibility – The Model P8400 Hemostatix
Thermal Scalpel Controller is compatible with the following
thermal scalpel blades:
•REF 7013-5810 – #10 Blade - Sterile Disposable - Quantity 24
•REF 7023-5810 – #10 Blade - Sterile Disposable - Quantity 10
•REF 7023-5812 – #12 Blade - Sterile Disposable - Quantity 10
•REF 7013-5815 – #15 Blade - Sterile Disposable - Quantity 24
•REF 7023-5815 – #15 Blade - Sterile Disposable - Quantity 10
3. FOOTSWITCH Compatiblity
•The Model P8400 Hemostatix Thermal Scalpel Controller is
compatible with the optional Footswitch (REF 7013-8410).
4. SYSTEM CHECKOUTS NOTE: The Model P8400 controller
unit is rated 100-240 VAC ± 10%, 50 - 60 Hz ± 1 Hz.
1.POWER ON
Turn on POWER to the system by placing the POWER
SWITCH located at the front lower left side of the controller
console in the UP or ‘l’ position.
The unit will briefly (less than 10 seconds) undergo a self-test.
Once the self-test is completed, the display will illuminate with
the default set-point temperature setting (70˚ C) and prompt the
user to connect the handle.
2.POWER OFF
Turn off POWER to the system by placing the POWER
SWITCH located at the front lower left side of the controller
console in the DOWN or ‘0’ position.
3.HANDLE INSERTION
With the console powered ON and the handle ON/OFF
switch in the OFF position, attach the handle cable to the
OPERATING INSTRUCTIONS

16 17
controller console by aligning the arrow on the handle cable
connector with the arrow on the mating front panel connector
and inserting it into the front panel receptacle.
4.BLADE INSERTION AND CALIBRATION
The sterile, disposable blade/hub assembly includes a magnetic
interface. The blade/hub should automatically seat fully into
the Disposable Handle with zero force required when the
blade/hub assembly is placed into the mating portion of the
Disposable Handle. In the unlikely event that the hub does not
fully seat, simply manually press the hub into handle until the
blade is firmly seated as shown. NOTE: The handle should be
in the “OFF” position before inserting the blade.
5.USING THE HEMOSTATIX THERMAL SCALPEL
With the console powered ON, the handle attached and a blade
inserted into the handle, VERIFY that the console display reads 70°
C.
With the handle switched OFF, the blade temperature can be
elevated or lowered in increments of 10° C by using the UP ‘▲’ or
DOWN ‘▼’ arrows on the side of the handle, or the front of the
controller unit, or by using the optional footswitch pedals (See
Optional Footswitch Controls). The handle temperature controls
work only when the handle is switched OFF.
Slide the yellow handle ON/OFF switch to the ON position to
expose the tangerine-colored dot. The handle is now activated and
the blade will be heated to the displayed temperature setting.
To activate the thermal COAG mode, depress the blue Thermal
COAG switch on the top of the handle. The blade temperature
will approximate 300° C in the thermal COAG mode.
NOTE: The handle must be energized for the COAG switch to
work.
Blade Fully Seated Blade Partially Seated
Gap
If there is a pause during blade insertion, an error message
may appear. Simply remove the blade assembly and reinsert.
Once the blade is successfully inserted into the handle, the
controller unit will begin calibrating the blade. Calibration
should be completed within 10 seconds. If calibration is
successful, the controller unit will display a message
indicating that the blade has been calibrated.
NOTE: DO NOT use any type of instrument (e.g. hemostats)
to insert the blade into the handle as this would damage the
blade’s imprinted circuitry and render it inoperable.
NOTE: Accurate calibration can only be achieved if the blade
is at room temperature when it is inserted into the handle. If
the blade becomes accidentally dislodged from the handle,
turn the handle OFF, dip the blade in sterile water to cool it
to room temperature, and then reinsert it.
If calibration is not successful, an error message will appear on
the controller unit’s display. In that case, remove the blade and
re-insert it into the handle in one continuous motion. If the
controller unit continues to display an error message that the
blade is not calibrated, insert another new blade repeating steps.
If calibration with a second blade is not successful, then the
controller unit is unable to read the blade via the handle. Replace
the handle and reinsert a blade.

18 19
6.USING THE OPTIONAL FOOTSWITCH
e footswitch has two modes: (1) CUT/COAG and (2) TEMP
UP/DOWN. Switching from mode (1) to mode (2) and vice-
versa is controlled by depressing the black MODE button on the
top of the footswitch.
When in the TEMP UP/DOWN mode, depressing and releasing
the left (yellow) pedal will decrease the set point temperature by
10° C; whereas, depressing and releasing the right (blue) pedal
will increase the set point temperature by 10° C.
When in the CUT/COAG mode, depressing and holding the left
(yellow) pedal will energize the scalpel blade to come to the
selected temperature. Similarly, depressing and holding the right
(blue) pedal will energize the scalpel blade to come to the COAG
temperature of 300° C.
UPGRADE FEATURES
1. MAINTENANCE PORT – Located on the rear of the unit, this
port allows easy diagnostic
access for Hemostatix
personnel as well as easy
future software upgrade access
via a RS232 computer port.
e maintenance port is
covered with a maintenance
port cover.
WARNING: e
maintenance port is to
be used by Hemostatix
personnel only. e port is
not to be accessed, for any
purpose, by the customer.
Any attempt to connect via
the maintenance port will
result in a termination of
any warranties that may exist and may damage the unit.
2. AUXILIARY OUTPUT – Also located on the rear of the unit,
this port is designed to power future Hemostatix add on modules.
e purpose of the auxiliary output connector is to potentially
oer other modules (i.e. irrigation, suction, etc.) that would
complement the existing Hemostatix technology.
WARNING: e auxiliary port is not to be utilized for any
other purpose other than to power equipment specically
designed by Hemostatix. Any attempt to plug, non
Hemostatix equipment into the output connector may result
in damage to the Hemostatix unit thereby terminating any
warranties and may result in an unsafe electrical condition,
increased electrical emissions, or decreased immunity of
the Hemostatix system. Anyone who connects additional
equipment via the auxiliary output terminal is therefore
responsible for conguring a medical system and is
responsible that the newly congured system complies with
the requirements of the system standards IEC 60601-1 and
IEC 60601-1-3. If the user has questions regarding any
connections to the AUX output port, they should contact
Hemostatix Medical Technologies.
SURGICAL USE & TECHNIQUES
1. CUTTING TEMPERATURES
•SKIN – e recommended temperature setting for skin
incisions is 70° C. For minimal scarring, make the initial skin
incision with the scalpel handle in the OFF position. Cutting
with the blade unheated will eliminate the possibility of
supercial skin scars due to contact with a heated blade (See
WARNINGS and PRECAUTIONS).
•OTHER TISSUES – For other tissues, the appropriate
temperature setting is typically between 180° C and 300° C.
2. INFLUENCE OF CUTTING SPEED ON HEMOSTASIS
In practice, the surgeon generally selects the lowest set point
temperature that will aord adequate hemostasis for the

20 21
maximum anticipated rate of tissue cutting, thereby minimizing
unnecessary (thermal) necrosis of tissue. The determination of
the appropriate set point temperature is determined by the
surgeon by raising the set point temperature until adequate
hemostasis is achieved. Alternatively, the surgeon can, at any
selected point temperature, modulate the speed of tissue cutting
according to the vascularity of the tissue being incised.
3. SEALING BLEEDERS
•The heat from the Hemostatix Thermal Scalpel blade will seal
most (less than 2mm in diameter) blood vessels as they are cut.
•For a vessel not sealed as it is cut, promptly use the blade’s heat
to seal it by exerting light pressure on the bleeder with the flat
side of the blade.
•For larger bleeders, activate the Thermal COAG mode by
depressing and holding down the blue Thermal COAG switch
(or optional footswitch pedal) and holding the flat side of the
blade on the bleeder until hemostasis is achieved.
4. MAINTAIN A DRY OPERATIVE FIELD
•The most effective use of the Hemostatix Thermal Scalpel
thermal energy such that bleeding does not begin. This is done
by making incisions using long, relatively slow, authoritative
strokes (rather than short, “choppy” strokes) to maintain
constant and meticulous hemostasis at every step, and prevent
the onset of bleeding.
•Bleeding vessels are sealed by the direct contact of the hot blade
to tissue, thus providing heat transfer to the tissue at the
bleeding site. Accordingly, if pools of blood occur from vessels
not sealed as they are cut, suction or sponge the area before
applying the Hemostatix Thermal Scalpel blade to seal the
bleeders. Heat from the blade dissipates in pools of blood and
cannot get through these pools to reach the tissue to seal the
bleeder. Pools of blood simply coagulate on the blade, thermally
insulating it.
5. CHANGING THE BLADE
If the blade becomes dull or the change blade message appears on
the controller display, switch the handle ON/OFF switch to o
and wait for the temperature display to turn Green indicating the
blade is safe to handle. Replace the dull or damaged blade with a
new blade.
CAUTION: To remove a blade from the handle, grab the
plastic hub and pull the blade straight out of the handle.
Bending, twisting or flexing the blade could damage the
blade contacts and retainers within the handle causing it
to no longer function.
CAUTION: Used blades are surgically sharp and may be
extremely hot to the touch. Always use a sponge, clamp or
hemostat to grasp the used blade. Always follow proper
sharps precautions when handling a blade and biohazard
disposal techniques when discarding a used blade.
6. CLEANING THE BLADE DURING USE
Clean any coagulated blood or tissue debris from a HOT blade by
LIGHTLY wiping the blade using DRY 4x4 gauze. Using WET
gauze will cool the blade causing the blood and coagulum to
adhere to the surface of the blade. Only light pressure is needed.
Excess pressure will result in the bending of the blade and the
subsequent damage to the blade electrical circuit.
NOTE: e Teon non-stick coating cleans most eectively
when hot. Best results are obtained using dry 4x4 gauze when
the blade is hot.
NOTE: If the blade becomes accidentally dislodged from the
handle, turn the handle OFF, dip the blade in sterile water
or saline solution to cool it to room temperature, and then
reinsert it. Accurate calibration can only be achieved if the
blade is at room temperature when it is inserted into the
handle.
CAUTION: Care should be taken not to bend the blade
while cleaning, insertion, or reinsertion as the heater leads
may become broken and the blade stop working.
CAUTION: Never use any type of abrasive pad to clean the
blades. e abrasives will damage the circuit and render
the blade unusable.

2223
MAINTENANCE
External Cleaning is the only controller maintenance that can be
performed by the user.
•e console may be wiped down with a cloth dampened with
alcohol, mild soap, or detergent. Take care not to get liquids
into the inside of the controller unit.
•DO NOT immerse the console.
•DO NOT use an abrasive cloth or cleaners, especially on the
display screen.
NOTE: Servicing the controller unit by other than and qualied
service personnel approved by Hemostatix Medical Technologies,
LLC renders the Warranty void. For any service or warranty
questions, please call Hemostatix Medical Technologies, LLC.
NOTE: Before cleaning the controller, detach the controller unit
from the AC power source.
SERVICING
e Model P8400 Hemostatix ermal Scalpel System consists of the
controller unit, a handle, and a blade. If a problem is encountered,
any of the three may be the cause; therefore, it is important when
returning a controller unit for servicing to also return the handle(s)
and blade(s) that were in use when the problem occurred.
NOTE: Servicing the controller unit by other than and qualied
service personnel approved by Hemostatix Medical Technologies,
LLC renders the Warranty void. Before returning a controller
unit for servicing, please call, please call Hemostatix Medical
Technologies, LLC to obtain a Return Material Authorization
(RMA) and instructions as to how and where to send the
controller unit and accessories.

2425
WARRANTY
HEMOSTATIX MEDICAL TECHNOLOGIES, LLC (HMT) warrants to the original
purchaser that reasonable care has been used in the manufacture of the MODEL P8400
HEMOSTATIX THERMAL SCALPEL SYSTEM (HTSS) CONTROLLER and that,
when properly used, it will be free from defects in material or workmanship for a period of
one (1) year after the date of shipment from HMT or any of its authorized distributors.
NOTE: Hemostatix scalpel handles and blades are warranted to be free from defects in
materials and workmanship for a period of SIXTY (60) days from the date of shipment
e sole and exclusive remedy with respect to any MODEL P8400 HTSS or any portion
thereof found within its warranty period not to meet these standards is that after return to
and examination by HMT, HMT will without charge at its option either repair or replace
that portion of the MODEL P8400 HTSS found to be defective. is warranty shall not
apply (a) if that portion of the MODEL P8400 HTSS has been repaired or altered by
anyone other than qualied service personnel approved by HMT or altered in any way
which, in HMT’s judgment, aects its usability or reliability; or (b) if the sterile lot or serial
number has been altered, eaced, or removed; (c) if the fault has been caused by abnormal
conditions of operation or misuse including, but not limited to: dropping the controller
unit; opening the controller unit; and/or permitting electrical contact with an active electro
surgical (e.g., Bovie) electrode; operating the unit within 1 m of another electrosurgical
controller unit; or, (d) if in the case of the scalpel blades or handles, the scalpel blades or
handles have been reprocessed and reused.
Except for the replacement of fuses, which can be accessed without opening the controller
unit’s enclosure, any warranty, implied or expressed, is considered void if the tamper-proof
seal on the controller unit’s enclosure is found to be broken. In all such cases, HMT’s
determination will prevail and any repairs or replacements, if requested, will be billed at
HMT’s prevailing normal rates. If so requested, estimates will be submitted before work is
started.
NOTE: An RMA# issued by HMT must be obtained before any part of the MODEL
P8400 HTSS is returned.
NOTE: Handles and blades being returned must be cleaned, sterilized, and packaged in
sterile packaging with labeling which verifies the sterility of the handle and/or blade prior
to return to HMT. Any handle and/or blade not properly cleaned, sterilized and packaged
as described in this warranty will be disposed of and no warranty will be in effect.
e foregoing express warranty, as conditioned and limited, is in lieu of and excludes all
other warranties not expressly set forth herein whether expressed or implied by operation of
law or otherwise including, but not limited to, any implied warranties or merchantability or
tness for particular purpose. HMT shall not be liable for any incidental or consequential
loss, damage, expense or liability direct or indirect with respect to this product. HMT
neither assumes nor authorizes any other person to assume for it any other or additional
liability or responsibility in connection with this product.
SPECIFICATIONS
Model P8400 Hemostatix ermal Scalpel Controller Unit
NOTE: e MODEL P8400 HEMOSTATIX THERMAL SCALPEL SYTEM is
suitable for continuous operation.
Patient Leakage Current
(From Patient Connection
to Earth)
≤ 100 microamperes AC – Normal Condition
≤ 500 microamperes AC – Single Fault Condition
≤ 10 microamperes DC – Normal Condition
≤ 50 microamperes DC – Single Fault Condition
Blade Temperature Settings • VARIABLE,setbyUSERanddisplayed
on front panel display as TEMPERATURE
SETTING ranging from 70° C to 300° C in
10° C increments.
Room Operating
Environment • 15°Cto30°C(Note:Bladetemperatureis
indexed from room temprature
• 30%-75%RelativeHumidity-Non-
condensing
• 700to1060hPA
Transport & Storage
Environment • -29°Cto+50°C
•10%-85%RelativeHumidity
•570to1060hPA
Moisture Protection • 7013-8400ControllerUnit-IPX0Rating
• 7013-8410Footpedal-IPX8
Console Size • Approximately7.0inx10.9in
Console Weight • Approximately7.25lbs.(3.3kg)withoutpower
cord
Power Requirements • 100-240VAC±10%
• 50-60Hz±1Hz
Power Input • 1A
Power Output • 60Wdc
Fuses • T2A,H250V(3ABSloBlo,2Amp,glass
body, 6.35 x 31.75 mm) (Quantity 2)
Power Cord • Approx.10ft.HospitalGrade
HEMOSTATIX
THERMAL SCALPEL
SYSTEM is classied as a
Type BF Applied Part, Class
I electrical device and is
certied to the following:
•IEC60601-1Ed. 3.1 (2012)
•IEC60601-1-2 Ed. 4.0 (2014)

2627
ADES
SPECIFICATIONS
Model P8400 Hemostatix Thermal Scalpel System Handle - Model 9050
MODEL P8400 HEMOSTA TIX THERMAL SCALPEL SYSTEM BL
SERIES 5810, 5812, and 5815.
Model P8400 Hemostatix ermal Scalpel System Blades are available in the
following congurations (similar to cold, stainless steel scalpel blades): No.10, No.
12, and No. 15 blades.
Model P8400 Hemostatix ermal Scalpel System Blades are provided sterile and
ARE NOT intended for reuse.
Handle Switches: Latching On/O, momentary ermal Coag & Tem-
perature switches
Initial Sterilization Ethylene Oxide Gas
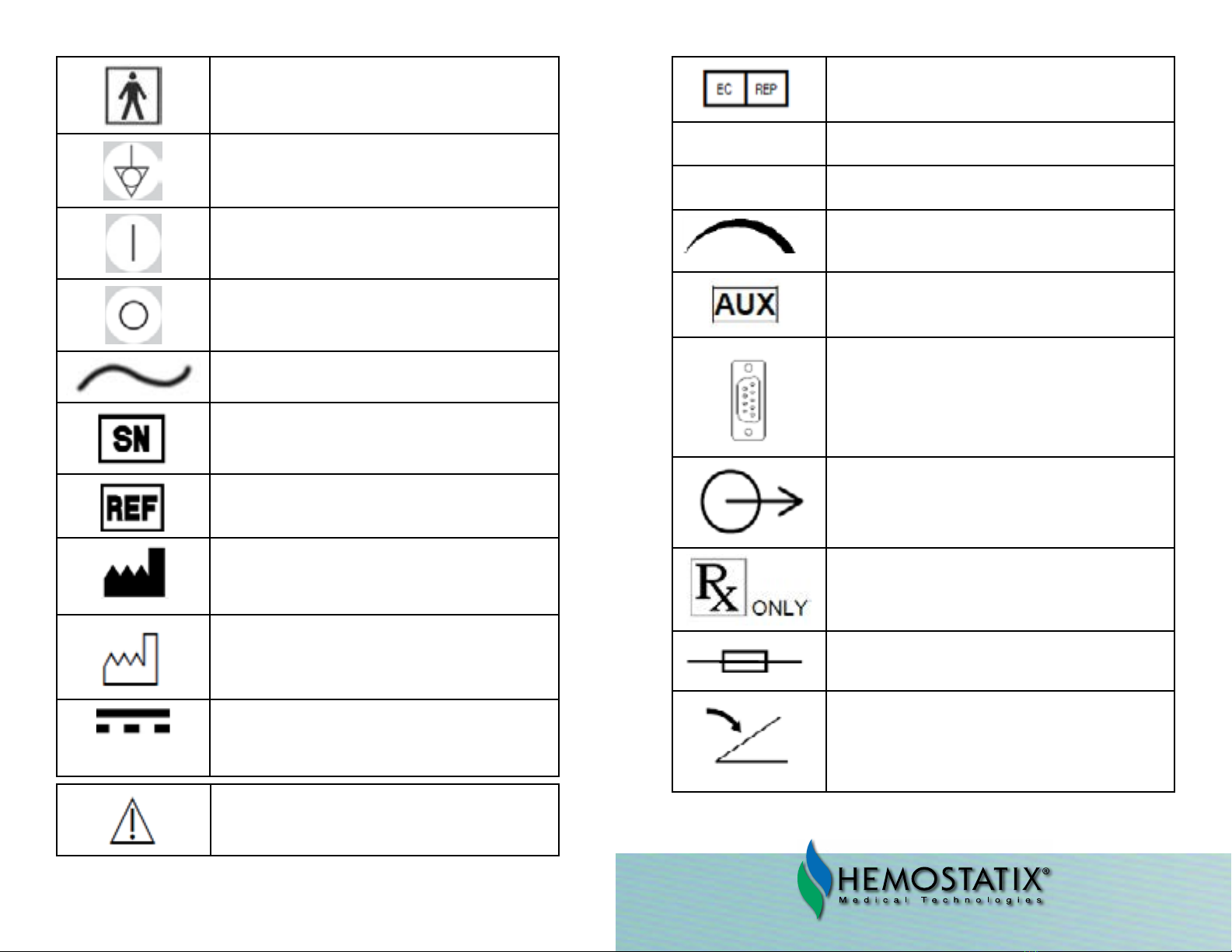
28 29
Type BF applied Part
Equipotentiality
On (power connection to mains)
O (power disconnection from the mains)
Alternating current
Serial number
Reference number
Manufacturer
Manufacturing date
Maximum DC Output
Caution
Authorized Representative in the
European Community
▼Temperature decrease
▲Temperature increase
Volume increase / decrease
Auxilliary output port
Auxilliary serial port
Output handle connector
CAUTION: Federal (U.S.A.) law restricts this device to
sale by or on the order of a physician.
Fuse
Foot pedal connector
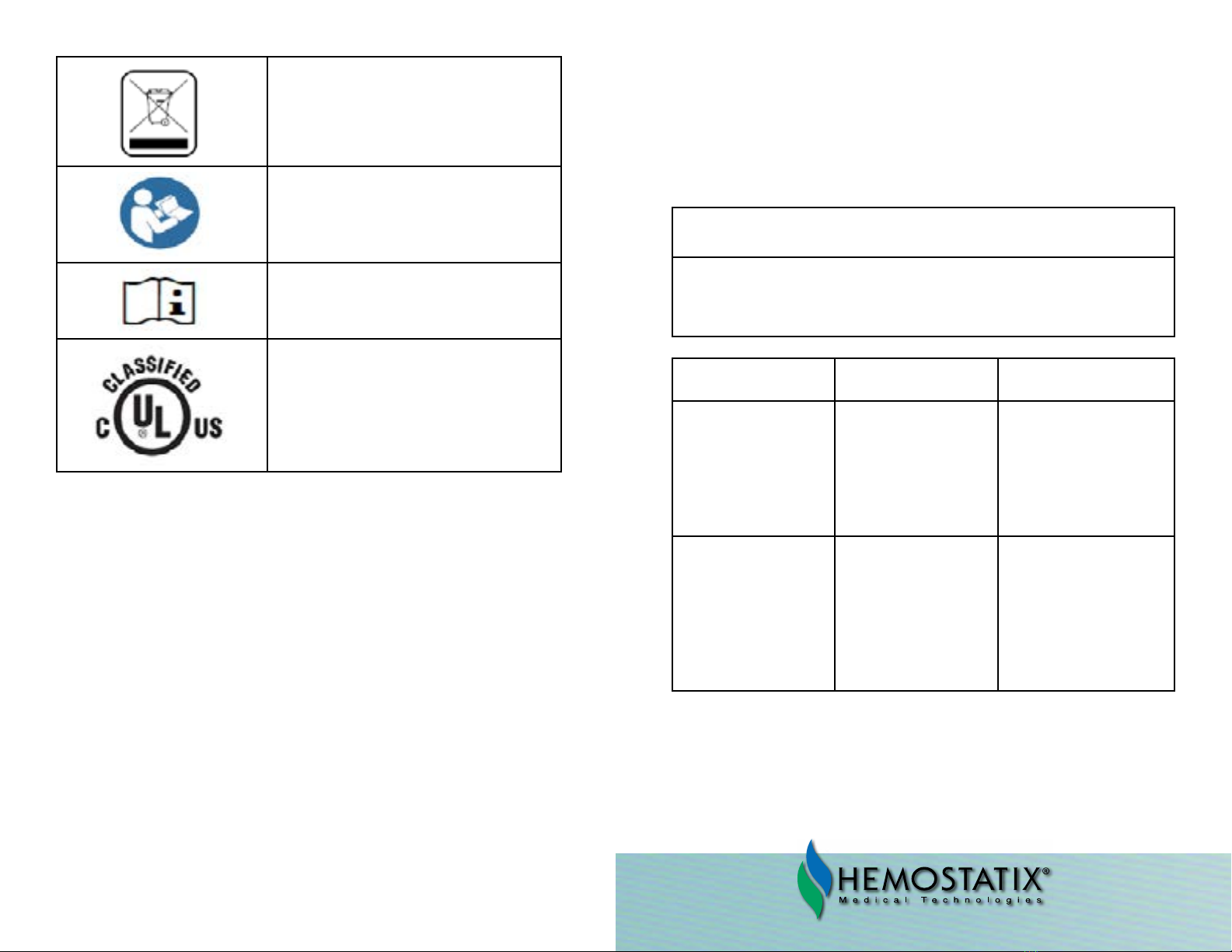
30 31
Do not dispose of this product in unsorted
municipal waste stream. Dispose of this prod-
uct according to Local Regulations.
Follow instructions for use
Consult instructions for use
Medical - General Medical Equipment
As to electrical shock, fire,and mechanical
Hazards only in accordance with:
ANSI/AAMI ES 60601-1:A1:2012
CSA CAN/CSA-C22.2 NO. 60601-1:14
GUIDANCE AND MANUFACTURER’S
DECLARATIONS
e Model P8400 Hemostatix ermal Scalpel System needs special precautions
regarding EMC and needs to be installed and operated according to the
information in the tables given below and portable and RF communications
equipment can aect the operation of the product.
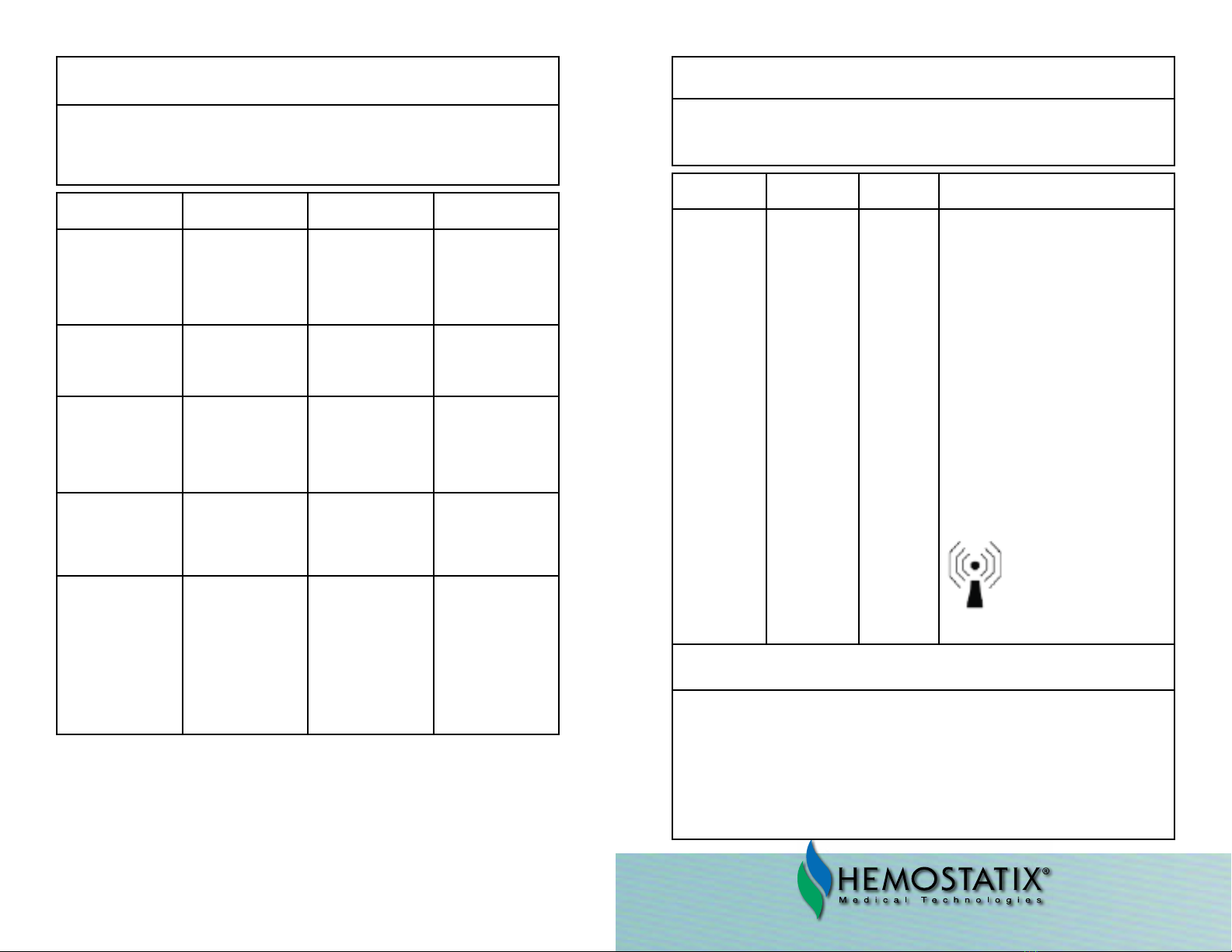
GUIDANCE AND MANUFACTURER’S DECLARATION
ELECTROMAGNETIC EMISSIONS
e Model P8400 Hemostatix ermal Scalpel System is intended for use in the
electromagnetic environment specied below. e customer or the user of the
Model P8400 Hemostatix ermal Scalpel System should assure that it is used in
such an environment.
Emissions Test Compliance Electromagnetic environ-
ment guidance
RF emissions Group 1 e device uses RF energy
only for is internal func-
tion. erefore, its RF
emissions are very low
and are not likely to cause
any interference in nearby
equipment.
RF emissions Class A e device is suitable for
use in all establishments
other than domestic and
those connected directly
to the public low-voltage
power supply network that
supplies buildings used for
domestic purposes.
3233
GUIDANCE AND MANUFACTURER’S DECLARATION
ELECTROMAGNETIC IMMUNITY
e Model P8400 Hemostatix ermal Scalpel System is intended for use in the
electromagnetic environment specied below. e customer or the user of the
Model P8400 Hemostatix ermal Scalpel System should assure that it is used in
such an environment.
Immunity Test Test Level Compliance Level Electromagnetic Envi-
ronmental Guidance
Electrostatic
Discharge
IEC 61000-4-2
± 6 kV Contact
± 8 kV Air
± 6 kV Contact
± 8 kV Air
Floors should be wood,
concrete or ceramic
tile. If oors are cov-
ered with synthetic
material, the relative
humidity should be at
least 30%.
Electrical Fast
Transient/Burst
IEC 61000-4-4
± 2 kV for mains
± 1 kV for signal leads
± 2 kV for mains
± 1 kV for signal leads
Mains power quality
should be that of a
typical commercial or
hospital environment.
Surge
IEC 61000-4-5
± 1 kV common mode,
AC mains
± 2 kV dierential
mode, AC mains
± 1 kV common mode,
AC mains
± 2 kV
dierential
mode, AC mains
Mains power quality
should be that of a
typical commercial or
hospital environment.
Power Frequency
Magnetic Field
IEC 61000-4-8
3 A/m 3 A/m Power frequency
magnetic elds above
those typically found
in commercial or
hospital environments
are acceptable.
Voltage dips, short
interruptions and volt-
age variations on AC
mains
IEC 61000-4-11
> 5% of nominal volt-
age for ½ cycle
40% of nominal volt-
age for 5 cycles
70% of nominal volt-
age for 25 cycles
> 95% of nominal
voltage for 5 seconds
> 5% of nominal volt-
age for ½ cycle
40% of nominal volt-
age for 5 cycles
70% of nominal volt-
age for 25 cycles
> 95% of nominal
voltage for 5 seconds
Mains power quality
should be that of a
typical commercial or
hospital environment.
For interruptions
longer than
10ms, power resets are
possible.
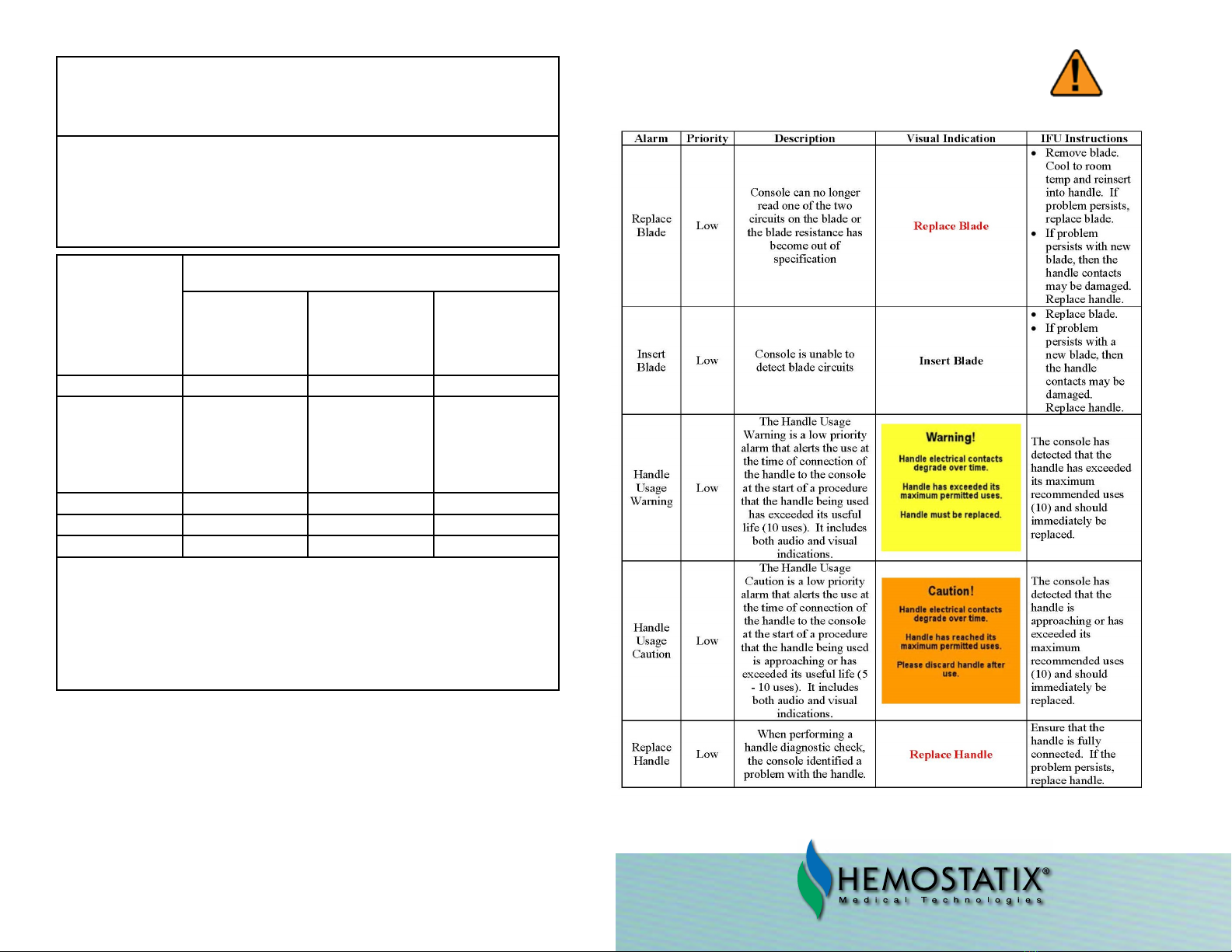
GUIDANCE AND MANUFACTURER’S DECLARATION
ELECTROMAGNETIC IMMUNITY
e Model P8400 Hemostatix ermal Scalpel System is intended for use in the electromagnetic en-
vironment specied below. e customer or the user of the Model P8400 Hemostatix ermal Scalpel
System should assure that it is used in such an
environment.
Immunity Test Test Level Compliance
Level
Electromagnetic Environmental Guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80
MHz
3 V/m
80MHz to
2.5GHz
3 Vrms
3 V/m
Portable and mobile RF communication
equipment should be used no closer to any
part of the device, including cables, than the
recommended separation distance calculated
from the equation applicable to the frequency
of the transmitter.
RECOMMENDED SEPARATION DIS-
TANCE:
d = 1.2√P
d = 1.2 √P 80 MHz to 800 MHz d = 2.4√P
800 MHz to 2.5 GHz where P is the maxi-
mum output
power rating of the transmitter in watts (W)
according to the transmitter manufacturer and
d is the recommended separation distance in
meters (m).
Field strengths from xed RF transmitters, as
determined by an electromagnetic site survey,a
should be less than the compliance level in
each frequency range.b
Interference may occur in the vicinity of
equipment marked with the following
symbol:
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: ese guidelines may not apply in all situations. Electromagnetic propagation is aected
by absorption and reection from structures, objects and people.
aField strengths from xed transmitters, such as base stations for radio (cellular/cordless) tele-
phones and land mobile radios, amateur radio, am and fm radio broadcast and tv broadcast cannot
be predicted theoretically with accuracy. to assess the electromagnetic environment due to xed rf
transmitters, an electromagnetic site survey should be considered. if the measured eld strength in the
location in which the model p8400 hemostatix thermal scalpel system is used exceeds the applicable
rf compliance level above, the model p8400 hemostatix thermal scalpel system should be observed to
verify normal operation. if abnormal performance is observed, additional measures may be necessary,
such as re-orienting or relocating the model p8400 hemostatix thermal scalpel system.
bOver the frequency range 150 khz to 80 mhz, eld strengths should be less than 3 v/m.
3435
RECOMMENDED SEPARATION DISTANCES BETWEEN
PORTABLE AND MOBILE RF COMMUNICATIONS EQUIPMENT
AND THE MODEL P8400 HEMOSTATIX THERMAL SCALPEL
SYSTEM
e Model P8400 Hemostatix ermal Scalpel System is intended for use in an electromagnetic
environment in which radiated RF disturbances are controlled. e customer or the user of the Model
P8400 Hemostatix ermal Scalpel System can
help prevent electromagnetic interference by maintaining a minimum distance between portable and
mobile RF communications equipment (transmitters) and the Model P8400
Hemostatix ermal Scalpel System as recommended below, according to the maximum output
power of the communications equipment.
Rated maximum
output power of
transmitter
W
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz
outside ISM bands
d=[3.5/V1]√P
80 kHz to 800
MHz in ISM
bands
d=[3.5/E1]√P
800 MHz to 2.5 GHz
d=[7/E1]√P
0.01 d=[3.5/3]√0.01 d=[3.5/3]√0.01 d=[7/3]√0.01
0.1 d=[3.5/3]√0.1 d=[3.5/3]√0.1 d=[7/3]√0.1
1 d=[3.5/3]√1 d=[3.5/3]√1 d=[7/3]√1
10 d=[3.5/3]√10 d=3.5/3√10 d=7/3√10
100 d=3.5/3√100 d=3.5/3√100 d=7/3√100
For transmitters rated at a maximum output power not listed above, the recommended separation
distance d in meters (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output rating of the transmitter in watts (W) according to the
transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: ese guidelines may not apply in all situations. Electromagnetic propagation is aected by
absorption and reection from structures, objects and people.
TROUBLESHOOTING BLADE / HANDLE
ERROR MESSAGES

36
TROUBLESHOOTING CONSOLE FAULT
MESSAGES
Hemostatix Medical Technologies, LLC
8400 Wolf Lake Drive, STE 109
Bartlett, TN 38133 USA
Telephone: +1 901-261-0012
Quality First International OU
Laki 30
12915 Tallinn
Estonia
© Hemostatix Medical Technologies, LLC All rights reserved
HEMOSTATIX® is a U.S.A. Registered Trademark (#2128414) of HEMOSTATIX®
Medical Technologies, LLC 3439100 REV BECR 00366– 07/2019
C 2797
This manual suits for next models
1
Table of contents
Popular Medical Equipment manuals by other brands
Welch Allyn
Welch Allyn Connex Integrated Wall System manual

Oxy-Pam
Oxy-Pam Inogen at Home Procedure guide

Neurosoft
Neurosoft Neuron-Spectrum-1/V Technical manual

Lowenstein Medical
Lowenstein Medical Azeer VENTIlogic LS instructions

Tomey
Tomey SP-100 Service manual

Miele
Miele PWD 8545 MD operating instructions

Codan
Codan Argus 718V End User Workbook & Competency Assessment

Novy
Novy CORNUAL CANNULATION SET manual

idi
idi Aspect 100-4 Q-CARD quick start guide

Zeiss
Zeiss MultiSEM 505 Series instruction manual

bewell connect
bewell connect MyThermo BW-CX10 user manual

Chemetron
Chemetron 560 Series Operation and maintenance manual