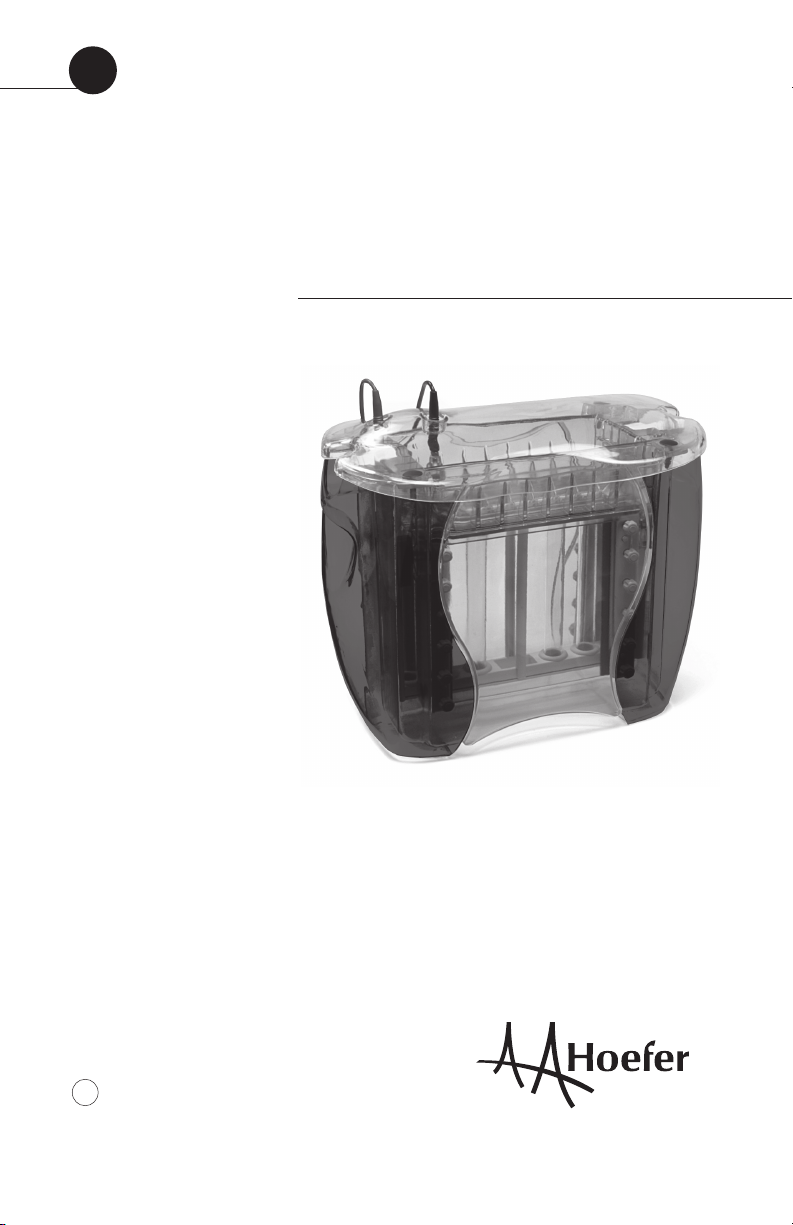
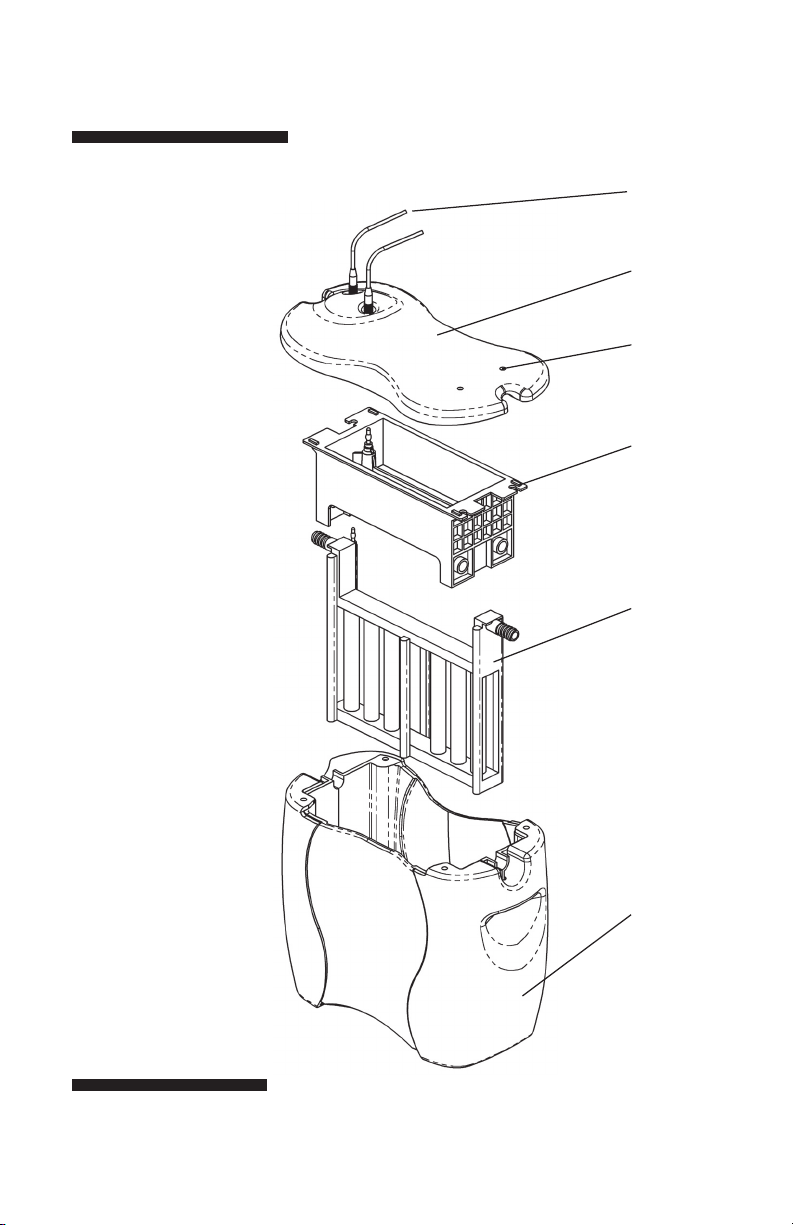
• p3
Important information
• The safety lid must be in place before connecting the power
leads to a power supply.
• Turn all power supply controls off and disconnect the power
leads before removing the safety lid.
• Circulate only water or 50/50 water/ethylene glycol through the
heat exchanger. Never introduce antifreeze or any organic solvent
into any part of the instrument. Organic solvents will cause
irreparable damage to the unit!
• Do not connect the heat exchanger to a water tap or any coolant
source where the water pressure is unregulated.
• Do not operate with buffer temperature above 45 °C. All plastic
parts are rated for 45 °C continuous duty. Circulate coolant
through the heat exchanger during electrophoresis to minimize
heating. Overheating will cause irreparable damage to the unit!
• Only accessories and parts approved or supplied by Hoefer, Inc.
may be used for operating, maintaining, and servicing this
product.
Informations importantes
• Le couvercle de sécurité doit être en place avant de brancher les
prises au générateur.
• Eteindre le générateur et débrancher les prises avant d’enlever le
couvercle de sécurité.
• Faire circuler seulement de l’eau ou 50/50 d’eau et d’éthylène
glycol dans l’échangeur vertical à cirulation d’eau. Ne jamais
utiliser d’anti-gel ou tout autre solvant organique avec cet
instrument. Les solvants organiques causeraient des dommages
irréparables à l’appareil.
• Ne pas connecter l’échangeur vertical à circulation d’eau à un
robinet ou quelque source de refroidissement dont la pression
n’est pas régulière.
• Ne pas utiliser avec un tampon à une température au dessus de
45 °C. Toutes les piéces en plastique sont prévues pour résister
à une température constante de 45 °C. Faire circuler l’eau dans
l’échangeur vertical durant l’électrophorèse pour minimiser
l’échauffement afin d’éviter des dommages irréparables à
l’instrument.
• Seulement les accessoires et piéces detachées approuvés ou
fournis par Hoefer, Inc. sont recommandés pour l’utilisation,
l’entretien et réparation de cet appareil.
�
�
�
�