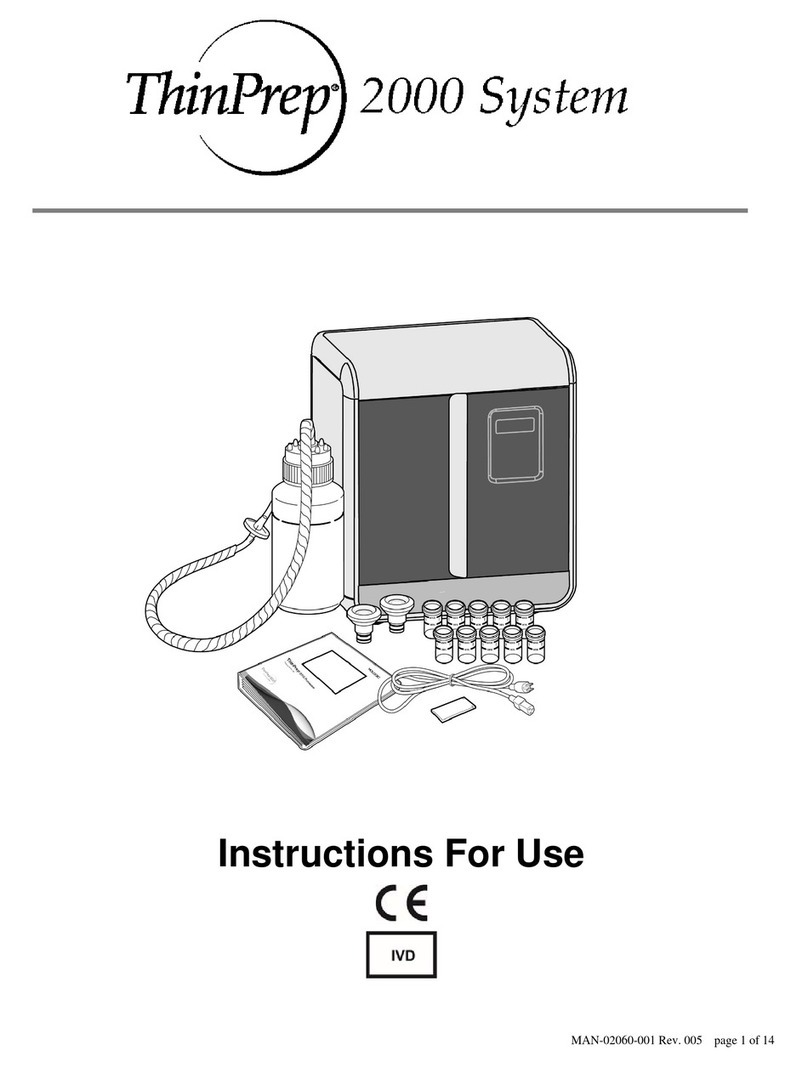
i
IMPORTANT: Read the entire manual before operating the Rapid fFN® 10Q System.
Hologic, Inc.
250 Campus Drive
Marlborough, MA 01752 USA
Tel: For Technical Support (USA and Canada)
1-800-442-9892
Fax: 1-508-263-2967
Tel: For Technical Support (Outside the USA and Canada)
Asia +852 3526 0718 Netherlands: 0800 022 6782
Australia: +61 2 9888 8000 Norway: 800 155 64
Austria: 0800 291 919 Portugal: 800 841 034
Belgium: 0800 773 78 Spain: 900 994 197
Denmark: 8088 1378 South Africa: 0800 980 731
Finland: 0800 114 829 Sweden: 020 797 943
France: 0800 913 659 Switzerland: 0800 298 921
Germany: 0800 183 0227 UK: 0800 032 3318
Ireland (Rep): 1 800 554 144 Rest of the world: 00800.800.442.9892
Italy: 800 786 308 Intl Fax number: 0041.21.633.39.10
©2019 Hologic, Inc. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any
means in whole or in part without the prior written permission of Hologic, Inc.
The Rapid fFN® 10Q Analyzer is covered by U.S. patent number 6,267,722. The Rapid fFN® 10Q QCette is covered by U.S. patent number Des. 432,244.
The Hologic logo, Rapid fFN® 10Q, and Rapid fFN® 10Q QCette are trademarks and/or registered trademarks of Hologic, Inc.
Printed in the USA AW-19875-001 Rev. 001 8-2019
Hologic BVBA
Da Vincilaan 5
1930 Zaventem
Belgium