ImpediMed SFB7 Technical manual
PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 1of 14
PERFORM Operating Document
Use and Cleaning of ImpediMed SFB7
PC-POD-CP-010-v01
Revision History
Version Reason for Revision Date
01 New POD November 26, 2015
Summary
The content of this PERFORM Operating Document (POD) provides guidelines for the
safe use and cleaning of the ImpediMed SFB7 as identified in the equipment inventory at
the PERFORM Center, Concordia University.

PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 2of 14
Table of Contents
SUMMARY------------------------------------------------------------------------------ 1
1DEFINITION OF TERMS ------------------------------------------------------- 3
2RELEVANT DOCUMENTS ---------------------------------------------------- 3
3INTRODUCTION --------------------------------------------------------------- 3
3.1 BACKGROUND -------------------------------------------------------------------------------------- 3
3.2 PURPOSE----------------------------------------------------------------------------------------------4
3.3 SCOPE ------------------------------------------------------------------------------------------------ 4
3.4 RESPONSIBILITY --------------------------------------------------------------------------------------4
4DEVICE COMPONENTS------------------------------------------------------- 4
5DEVICE CALIBRATION-------------------------------------------------------- 5
6MODES & PREDETERMINED MEASUREMENT SETTINGS ------------- 6
6.1 BIS MODE -------------------------------------------------------------------------------------------- 6
6.2 SFBI MODE------------------------------------------------------------------------------------------- 7
6.3 PREDETERMINED MEASUREMENTS SETTINGS ----------------------------------------------------- 7
7CONTRAINDICATIONS FOR USE------------------------------------------ 7
8PRELIMINARY INSTRUCTIONS---------------------------------------------- 8
9PREPARATION PRIOR TO TEST -------------------------------------------- 8
10 TEST PROCEDURE ----------------------------------------------------------- 8
11 DATA RETRIEVAL -----------------------------------------------------------10
11.1 READING DATA FROM DEVICE ------------------------------------------------------------------- 10
11.2 EXPORTING DATA FROM DEVICE ---------------------------------------------------------------- 10
12 SAFETY AND CLEANING -------------------------------------------------12
12.1 SAFETY---------------------------------------------------------------------------------------------- 12
12.2 CARE AND MAINTENANCE----------------------------------------------------------------------- 13
13 TROUBLESHOOTING------------------------------------------------------14
APPENDIX I:POD TRAINING RECORD FORM

PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 3of 14
1Definition of Terms
2Relevant Documents
This POD is governed by the following Concordia University policies, SOPs, and PODs:
•PC-SOP-GA-007 “General Access to PERFORM Centre”.
•PC-SOP-GA-009 “Emergency Response Procedures at the PERFORM Centre”.
•PC-SOP-CP-001 “Cardio-Pulmonary Suite – Access, Use, and Training of
Personnel”.
•PC-POD-GA-001 “PERFORM Centre Booking System for Facilities and
Equipment”.
3Introduction
3.1 Background
Body composition can be assessed using various techniques from field to more clinical
tests. The ImpediMed SFB7 is a portable battery powered device that offers a rapid,
reliable, non-invasive, and cost effective means of measuring body composition (i.e. fat-
Standard Operating
Procedure (SOP)
SOP’s at PERFORM are any operating document that require a
full review process and approval by the SD.
PERFORM operating
document (POD)
Operating documents that are specific to an instrument or
technique that require approval by area managers.
Protocol Methods that are developed by users on specific
instruments/equipment
Users
Person using space or equipment at the PERFORM
Centre that has received adequate technical and safety
training.
Supervisor
Knowledgeable person regarding all or an aspect of a project or
program and is familiar with PERFORM’s best practices, that is
responsible for ensuring that users conduct their activities in a
safe manner and within scope of the project.
PERFORM Employees Concordia employee that has been assigned to PERFORM.

PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 4of 14
free mass, fat mass, intra-/extracellular fluid) of participants. Fat contains little water
while the lean compartment has most of the body’s water. Therefore, when the current
encounters fat, there is more resistance. By measuring how easily currents move
through the body, body fat can be estimated. This technique is known as bioelectrical
impedance analysis.
3.2 Purpose
The objectives of the current POD are to 1) outline the procedure for using ImpediMed
SFB7; 2) provide a set of standard practices for safe operation and a training guide for
new users of the systems at the PERFORM Centre, Concordia University; and 3) outline
the procedure of cleaning/disinfecting of auxiliary items used for this device.
3.3 Scope
This POD applies to all users and supervisors using the ImpediMed SFB7 at the
PERFORM Centre, Concordia University. Any other document other than this POD is
out of scope for this operating procedure.
3.4 Responsibility
It is the responsibility of all users and supervisors to ensure that this POD is followed.
4Device Components
1= BIA electrodes 5= Power module for recharging the battery 9= Stylus pen
2= Lead set 6= Main power cable 10= Microfiber cloth
3= Alligator clips (4) 7= Crossover network cable
4= Test cell for calibration check 8= CD-ROM

PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 5of 14
5Device Calibration
5.1 Plug power cable into outlet and back of device to be sure it is charged.
Battery Status Indicator
5.2 Turn device on by pressing the On/Off button on the front panel of the
unit. After a couple of seconds the device will display the ImpediMed logo
and will go to the main menu.
5.3 Connect each colored lead to corresponding color on back of device. Make
sure the arrow on lead is in line with notch on device.
5.4 Remove alligator clips from lead wires.
5.5 Connect leads to the test cell according to color coding.

PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 6of 14
5.6 Press “Test” on screen using stylus pen.
NOTE: Stylus should always be used when making selections on touch screen.
5.7 Press “Start”. Screen will display “Calculating”.
5.8 Should it read “pass”, device is ready to use
NOTE: Should it read “fail”, contact area supervisor.
5.9 Click “More” and when the graph is displayed click “More” again. “Rzero”
and “Rinf” should read “Rzero: 604 (± 7 ohms)” and “Rinf: 403 (± 5 ohms)”.
5.10 Press “Back” 4 times.
5.11 Disconnect the leads from test cell and replace the alligator clips.
NOTE: Failing to adhere to the above conditions may affect your results.
6Modes & Predetermined Measurement Settings
The ImpediMed SFB7 offers two modes of functions: bioimpedance spectroscopy (BIS),
and selected frequencies (SFBI).
6.1 BIS Mode
This mode measures BI paramaters over a frequency range of 4-1000 kHz with 256
data points defining the BIS mode as a true BI spectroscopy. On-screen graphs display
the measured data in the form a cole-cole plot.
Reactance vs. Resistance: The Cole-Cole plot gives a visual representation
of the measurement and is used to indicate a valid measurement has been
taken. The absence of scattered data points and well fit Cole-Cole plot
signifies noise free data and that a valid measurement has been performed.
Resistance vs. frequency plot and reactance vs. frequency plots
are visual representation of the measured bioimpedance data.

PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 7of 14
In addition the characteristic frequency for the subject is determined as well as total cell
membrance capacitance. These estimates are then used in algorithms to give body water
and fat-free mass.
6.2 SFBI Mode
This mode measures BI parameters over a frequency range of 4-1000 kHz. There are 8
selectable frequencies of which 5 are fixed and 3 are user definable. The 5 fixed
frequencies are: 5, 10, 50, 100, 500 kHz, and the 3 user definable frequencies may be
any in the range of available frequencies. A user may also specify the number of
measurements to be taken, with a specified measurement interval.
The SFBI function is included to allow a user to compute and present estimates of body
composition using published algorithms.
6.3 Predetermined Measurements Settings
Touching the arrow button next to the “Measurements” selection box allows the
selection of single or continuous measurements, or several measurements made spaced
at a selected interval of time. In continuous mode, another measurement is commenced
immediately after the previous measurement has been completed. In interval mode,
another sequential measurement is made after the interval of time selected (in seconds).
For Interval measurement setting, the interval between measurements can be selected
by touching the arrow buttons next to the “Interval” edit box or by using the keypad
(see Start up Screen section) that is selected by touching the “Interval” edit box.
For Interval and Continuous measurement settings, the number of measurements can
be selected by touching the arrow buttons next to the “Number” edit box or by using
the keypad (see Start up screen section) that is selected by touching the “Number” edit
box.
7Contraindications for Use
This device must not be used on participants with the following devices/conditions:
•Active implanted medical devices (e.g. cardiac pacemaker, defibrillators, or
participants connected to electronic life support devices)

PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 8of 14
•Undergoing external defibrillation
•Pregnant
8Preliminary Instructions
8.1 Participant should respect the following instructions in order to minimize
changes in the body’s hydration status (as this can over or underestimate
values):
8.1.1 Excessive exercise should be avoided 2 hours prior to measurement
to avoid changes in vascular perfusion, temperature, cutaneous blood
flow, vasodilation, and fluid losses.
8.1.2 Refrain from drinking excessive alcohol within 12 hours prior to BIA.
8.1.3 Also, the following situations affect body water concentration:
i. just prior, during, just after menstruation
ii. use of diuretics
iii. renal or heart failure
9Preparation Prior to Test
9.1 Evaluator must plug device at least 15 minutes in advance to ensure battery
is charged for evaluation.
9.2 Check that participant has followed preliminary test instructions.
NOTE: Device will automatically shut-off after a period of inactivity.
10 Test Procedure
10.1 Ask participant to turn off mobile phone.
10.2 Ask participant if they want to go to the washroom. Measure height
(nearest 0.5 cm) and weight (nearest 0.1 kg).
10.3 Remove all jewelry, stockings, pantyhose, and/or socks.
10.4 Lie participant on their back on table feet shoulder width apart.
Measurement should be taken within 10 minutes of the participant lying
down. There is evidence that impedance values rise sharply within the first
10 minutes after the participant assumes the supine position and then
continue to rise more gradually for up to 4 hours.
10.5 Extend their arms by their side with palms down, legs slightly apart (if
necessary, place towel between participant’s legs or arms and torso in
order to prevent skin-to-skin contact and reduced impedance).
10.6 Using the anatomical locations shown below shave the sites, if necessary.
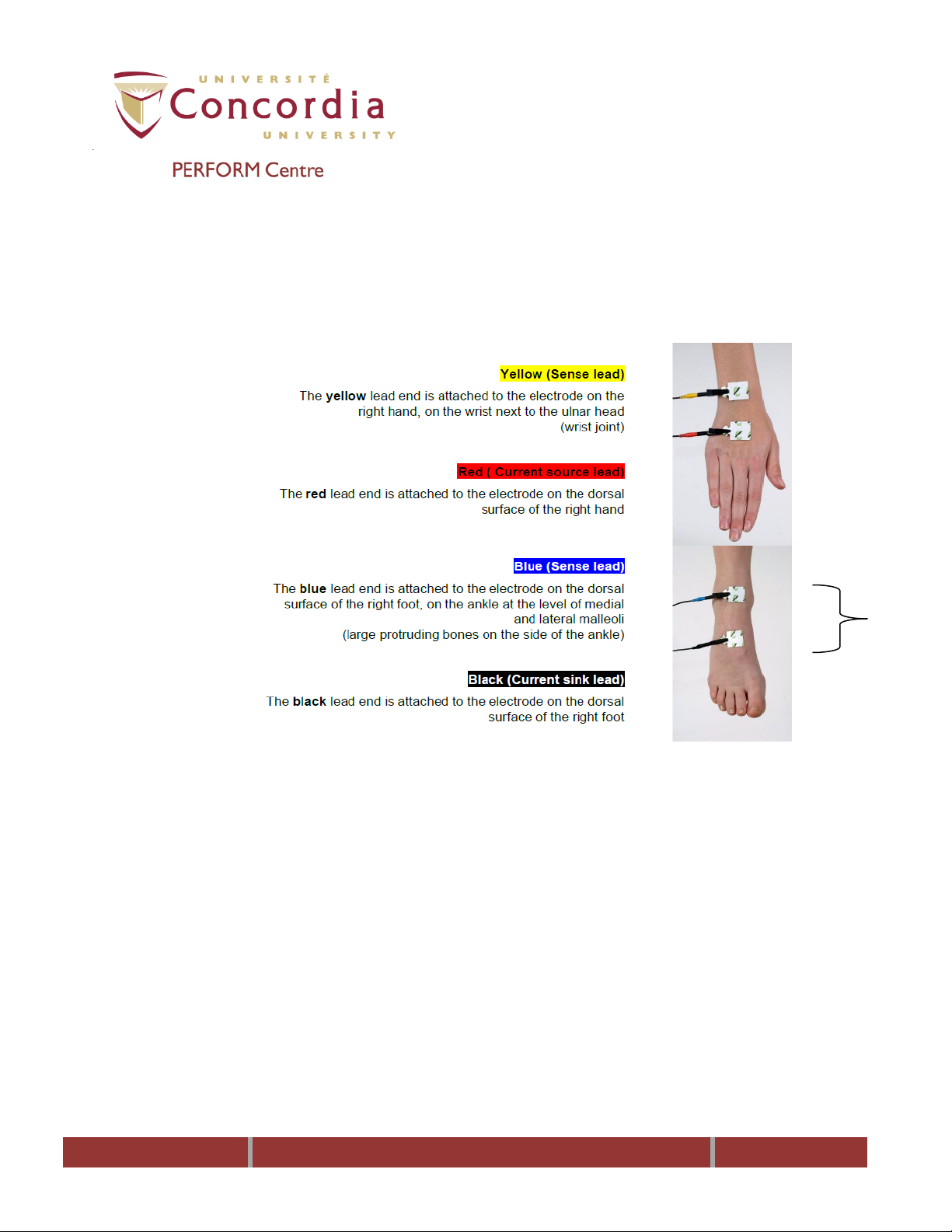
PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 9of 14
NOTE: Measurements can be done on either the right or left side, but do not mix sides.
Consistent measurement practices and electrode placement are important to obtain
accurate results.
10.7 Clean the sites with an alcohol swab.
10.8 Allow sites to dry for 30 seconds before placing electrodes.
10.9 Place electrodes, with tabs pointing away from body, ensuring that they are
5 cm apart measured from the center to center (a 1-cm displacement of
electrodes can result in a 2-percent change in resistance). Do not press
down on the electrodes too firmly.
10.10 Attach lead wires to electrode tabs.
NOTE: Ensure that the metallic part of clip is in direct contact with the conductor side
(underside) of the electrode tab and that the clips are aligned to the centers of the
electrode tabs.
10.11 Click “Measure” in main menu.
10.12 Click on the box under “File Name” and enter participant ID and press
“Ok”.
10.13 Click on “Edit” under “Patient Details”.
10.14 Enter information and click “Ok”.
5 cm

PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 10 of 14
10.15 Predetermined measurement settings (e.g. number of measures to be
taken and at what interval) are set to the following, but can be modified at
this stage:
Measurement: interval
Interval(s): 10
Number: 3
10.16 Click “Measure”.
10.17 Click “Start”. Once measurements are completed (based on
predetermined settings) the device will say “Ready” at the top. Data is
automatically saved.
10.18 Press “Exit”, then “Back” 2 times to go back to the main menu.
11 Data Retrieval
11.1 Reading data from device
11.1.1 Press “Files” from the main menu.
11.1.2 Use the arrow buttons to navigate the file list.
11.1.3 The selected file can be viewed by touching “View File”.
11.1.4 Press “More…” button will display a Cole-Cole plot.
11.1.5 Files can be sorted by name or date and time. Touch either the “Name
Sort” or “Date Sort” button.
11.2 Exporting data from device
NOTE: When the file storage memory on the device is full, the device stops taking
measurements and displays the “File system full remove files to make space” screen. In
order to continue taking measurements, transfer files on to a PC loaded with Biolmp
Body Composition Analysis software or delete the files which are not required.
11.2.1 To transfer data to the Analysis Software you need to do these three
steps first:
Step 1: Installing the AMD-Tek or Swann USB-Ethernet adapter. Safety, Care and
Maintenance:
1. Insert the supplied AMD-Tek or Swann USB-Ethernet adapter into a spare
USB slot on your computer.
2. Your computers operating system will automatically detect and install the
drivers for the adapter.

PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 11 of 14
3. Restart your computer to allow the adapter to be fully installed.
4. Once your computer has restarted insert your ImpediMed software CD-Rom
into your computers CD-Rom drive.
5. Select your language.
6. Click the Set up the USB Ethernet adapter button.
7. This window will display all USB-Ethernet adapters that have been installed on
your computer. Click the USB to fast Ethernet converter inside the network
adapters found window and click the Next button to start auto-configuring
your USB-Ethernet converter.
8. Click the Finish button to continue the auto-configuration.
9. Click the OK button and restart your computer when the above prompt
window appears to finalize the USB-Ethernet adapter setup.
Step 2: Installing your BioImp software:
1. Once your computer has restarted re-insert your ImpediMed software CD-Rom
into your computers CD-Rom drive.
2. Select your language.
3. Click the Install “BioImp” button.
4. Click the “Next” button to continue or “Cancel” to exit the software setup.
5. Click the Yes button to accept the software license agreement and continue with
installation and follow the onscreen instructions to finish installation of your
software.
Step 3: Connecting your SFB7 device to your PC:
1. Turn off your SFB7 and shutdown your PC.
2. Attach the RED Ethernet crossover cable from your device to your PC.
3. Turn on your PC and once the PC is booted turn on your Imp SFB7 device.
4. Your PC will now detect the new network connection and you are ready to
upload files from the SFB7 to your software or computer.
NOTE: BIS and SFBI measurement data is stored on board, but only BIS measurement
data is retrievable via Ethernet.
NOTE: Uploaded files are automatically deleted from the Imp SFB7. Sophisticated file
transfer ensures that each file is transferred, confirmed as received, before deletion.

PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 12 of 14
12 Safety and Cleaning
12.1 Safety
12.1.1 Only use the power adaptor (Amtex 9940) that is supplied with this
device. The use of any other power adaptor may expose the patient to
the risk of electrocution.
12.1.2 Do not connect the Imp SFB7 device to:
12.1.2.1 Patients with active implanted medical devices, e.g
cardiac pacemakers, defibrillators or patients connected to
electronic life support devices.
12.1.2.2 Patients undergoing external defibrillation.
12.1.3 Do not plug SFB7 leads to any mains power outlet/point.
12.1.4 Do not use or operate device in the presence of strong
electromagnetic fields.
12.1.5 The ImpediMed SFB7 should be unplugged from the recharging unit
before use to avoid possible noise contamination of the measurement.
12.1.6 The ImpediMed SFB7 has yet to be clinically validated for use on
pregnant patients.
12.1.7 Only use ImpediMed electrodes.
12.1.8 Avoid placing an electrode on an irritated skin site.
12.1.9 If skin irritation occurs seek professional advice.
12.1.10Allow skin to dry thoroughly before placing electrodes on skin.
12.1.11Do not connect alligator clips to patient’s skin.
12.1.12Do not mix single use and reusable or different brands of electrodes.
12.1.13Do not cut the electrode, use whole electrode only.
12.1.14Do not use extra gel with solid gel electrodes.
12.1.15Do not leave the electrodes attached to the skin for longer than 1
hour.
12.1.16Use only the cable leads supplied by ImpediMed Limited with the Imp
SFB7. The use of non ImpediMed leads can cause damage to the device
or give an incorrect reading.
NOTE: There are no user adjustable parts in the device, do not disassemble unit.

PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 13 of 14
12.2 Care and Maintenance
12.2.1 Care of the product:
12.2.1.1 When in use always keep the SFB7 in the carry
case.
12.2.1.2 Clean the SFB7 with a dry, clean cloth only.
12.2.1.3 Avoid exposure to water, impact and excessive
heat or direct exposure to sunlight.
12.2.2 Care of the leads:
12.2.2.1 Clean leads with a damp cloth if required.
12.2.2.2 Wherever possible leads should remain connected
to the device.
12.2.2.3 Unnecessary removal of the leads from the device
may reduce lead life.
12.2.2.4 Ensure that the lid of the case does not close on
the leads.
12.2.2.5 Do not wind the leads tightly, crinkle, or twist the
leads, as this may cause the fine wires inside to break.
12.2.2.6 If the leads appear to be damaged, contact
ImpediMed or a licensed distributor for replacements.
12.2.3 Care of the electrodes:
12.2.3.1 The electrodes are for single use. Please discard
after use.
12.2.3.2 Reseal the electrode pouch after use.
12.2.3.3 Unused electrodes should remain in the supplied
pouch and in a cool dry place to prevent electrode gel
from dehydrating.
12.2.3.4 Use by expiry date.
12.2.3.5 Do not use an electrode if the conductive adhesive
is dry and no longer pliable or sticky. This may result in
inaccurate measurements.
12.2.4 Care of the touch screen:

PC-POD-CP-010-v01
PC-POD-CP-010-v01 Printed copies are not controlled. Page 14 of 14
12.2.4.1 Always use the stylus pen provided to operate the
touch screen. Use the rubber end of the stylus pen to
operate Imp SFB7 touch screen.
12.2.4.2 If necessary clean the touch screen with a soft
damp cloth, and do not use any liquids directly.
13 Troubleshooting
If the user encounters any problems, prior, or during testing, they can refer to the table
below.
Internal software fault.
The device stops responding
to touch screen commands.
Power light is blinking.
Or
Power light is red.
Or
Power light is orange.
Normal behaviour.
Refer to section "Battery Status
Indicator."
1. Power the device off. Power the
device on.
2. Report the fault to ImpediMed.
1. Ensure the leads have been correctly
fitted according to the colour coding.
2. Ensure the leads are inserted properly
into the lead sockets.
3. Ensure the leads are not damaged or
tangled.
4. Ensure the lead are properly inserted
into the alligator clips.
5. Ensure the alligator clips are securly
attached to the electrodes.
6. Ensure the electrodes are fresh.
7. Perform the calibration check to
determine if the device is faulty.
8. If performing the calibration check,
ensure the colour coding is correct and
the leads are pushed snugly into the test
cell.
The message "Out of Range
Check Leads and Electrodes"
is displayed on the screen.
Or
The reading is obviously
incorrect.
The device is not correctly set up.
1. Attach external power pack and try
again.
2. If external power pack is attached,
check that power pack is plugged in and
switched on.
1. Battery power is low.
2.No external power.
No display when SFB7 is
switched ON.
Problem
Possible Cause
Solution

PC-POD-CP-010-v01
PC-POD-CP-001-v01 Printed copies are not controlled. APPENDIX I
APPENDIX I
POD Training Record Form

PC-POD-CP-010-v01
POD Title
Use and Cleaning of ImpediMed SFB7
SOP Code
Ownership Document type Area SOP Number Version
PC POD CP 010 V01
Training Record
Full Name
Institution
Contact
(email or phone number)
Signature
Sign here
Date
Other manuals for SFB7
1
Table of contents
Other ImpediMed Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Kinetec
Kinetec Maestra manual

Hitachi
Hitachi EUP-VV531 instruction manual

Trulife
Trulife Pressurecare Series Instructions for use

OBDSPACE TECHNOLOGY
OBDSPACE TECHNOLOGY ANCEL AD610 manual

human care
human care 90702 user manual

Fresenius Medical Care
Fresenius Medical Care GranuFlo 450368-03 Operator's manual