ImpediMed SOZO User manual

ImpediMed – Feb 2018 Page 1 LBL-507-en REV C
SOZO
Instructions For Use
ImpediMed – Feb 2018 Page 2 LBL-507-en REV C
ImpediMed Limited
ABN 65 089 705 144
Unit 1
50 Parker Court
Pinkenba Qld 4008
Australia
Phone: + 61 7 3860 3700
Fax: + 61 7 3260 1225
Email: enquiries@impedimed.com
Website: http://www.impedimed.com/
EC REP
MediMark Europe Sarl
11 rue Emile ZOLA,
B.P. 2332, 38033 Grenoble Cedex 2
France 0086
For EU Customers: All products at the end of their life may be returned to ImpediMed for
recycling.
For patent(s) and/or patent application(s) see: https://www.impedimed.com/patents/
For assistance with product set up
please review the help videos at
https://help.hellosozo.com. For other
assistance, or to report product issues,
please contact ImpediMed by email at
61 7 3860 3700.

ImpediMed – Feb 2018 Page 3 LBL-507-en REV C
Contents
Minimum Requirements..............................................................................................5
Tablet..............................................................................................................................................5
SOZO App......................................................................................................................................5
Introduction .................................................................................................................6
The SOZO Device..........................................................................................................................6
Bioimpedance Spectroscopy (BIS) ................................................................................................6
Components of the SOZO System.............................................................................7
Safety Instructions......................................................................................................8
Signs and Symbols.........................................................................................................................8
Intended Use..................................................................................................................................9
Contraindications............................................................................................................................9
Warnings ........................................................................................................................................9
Precautions.................................................................................................................................. 10
Use and Storage Conditions ....................................................................................................... 11
Environmental Operating Conditions .......................................................................................... 11
Environmental Transport and Storage Conditions...................................................................... 11
Location for Use .......................................................................................................................... 12
Setting Up the SOZO Device and Stand..................................................................13
Setting Up the SOZO Device without Stand............................................................ 15
Step 1: Connecting SOZOtouch and SOZOstep......................................................................... 15
Step 2: Connecting the Tablet Stand .......................................................................................... 15
Step 3: Connecting Power Supply............................................................................................... 15
SOZOhub installation................................................................................................17
Pairing Tablet and SOZO App to SOZO System.....................................................18
Step 1 .......................................................................................................................................... 18
Step 2 .......................................................................................................................................... 18
Step 3 .......................................................................................................................................... 18
Initial setup of SOZO app............................................................................................................ 18
Pairing the SOZO app with the SOZO system............................................................................ 20
Preparing the patient.................................................................................................22
Standing vs. Seated positions..................................................................................................... 22

ImpediMed – Feb 2018 Page 4 LBL-507-en REV C
Optimal readings ......................................................................................................................... 22
Standing Position ........................................................................................................................ 22
Seated position............................................................................................................................ 23
Using the SOZO App.................................................................................................24
Overview...................................................................................................................................... 24
Start-up........................................................................................................................................ 24
Logging In.................................................................................................................................... 24
Creating/Accessing Patient Profiles............................................................................................ 26
Taking Measurements................................................................................................................. 29
Weight Entry................................................................................................................................ 30
Foot Placement ........................................................................................................................... 31
Hand Placement.......................................................................................................................... 31
Measurement Time ..................................................................................................................... 32
Cole Plots.................................................................................................................................... 32
Viewing Historical Measurements and Patient Trending............................................................. 33
Changing Device Settings........................................................................................................... 36
Selecting SOZO device within the SOZO app ............................................................................ 36
Indications for Use.................................................................................................... 37
SOZOthrive for Lymphoedema ................................................................................................... 38
SOZOthrive Module Results........................................................................................................ 39
SOZOpro for Body Composition Measurements......................................................................... 41
SOZOpro Module Results ........................................................................................................... 41
SOZObeat for Fluid Monitoring Measurements .......................................................................... 43
SOZObeat Module Results ......................................................................................................... 43
Personal Data ............................................................................................................44
Care and Maintenance ..............................................................................................45
Care of the Product ..................................................................................................................... 45
Self-Test Function ....................................................................................................................... 45
Repairs ........................................................................................................................................ 45
Technical Support ....................................................................................................................... 46
Troubleshooting........................................................................................................ 47
ImpediMed Product Warranty...................................................................................51
Regulatory Statement ...............................................................................................52
Product Specifications..............................................................................................53
Safety and Electromagnetic Compatibility (EMC) Information.............................. 54

ImpediMed – Feb 2018 Page 5 LBL-507-en REV C
Minimum Requirements
Tablet
The Samsung Galaxy Tab A (model SM-T580) will be provided to operate the SOZO device.
For more product details, please review www.samsung.com or the tablet’s User Guide.
Note: The Bluetooth®word mark and logos are registered trademarks owned by Bluetooth
SIG, Inc. and any use of such marks by ImpediMed is under license. Other trademarks and
trade names are those of their respective owners.
SOZO App
The supplied tablet is preloaded with ImpediMed’s SOZO app for Android. The tablet and app
will need to be paired via bluetooth to the SOZO device at initial setup. The app must also have
network access over Wi-Fi®to the SOZOhub software to function.

ImpediMed – Feb 2018 Page 6 LBL-507-en REV C
Introduction
The SOZO Device
SOZO: A bioimpedance spectroscopy device for use on human patients, utilizing ImpediMed’s
patented BIS technology in a user-friendly format to allow quick and accurate measurement of
fluid levels. This can be used as an “early warning” monitor for determining changes in the fluid
status of patients with fluid management problems.
Bioimpedance Spectroscopy (BIS)
Bioimpedance spectroscopy (BIS) is the only non-invasive technology available that has the
potential to accurately measure body water volumes. BIS mode measures bioimpedance
parameters over a frequency range of 3 - 1000 kHz with 256 data points. Comparison of the
data collected within that frequency range enables calculation of extracellular, intracellular and
total body water. With additional patient-specific data, further calculations can be made to
determine other body composition results. On-screen graphs also display the measured data in
the form of a Cole plot.
The SOZO device and SOZOthrive module offers rapid, non-invasive, inexpensive comparison
of impedance ratio’s between normal limbs and limbs at risk of lymphoedema. The instruments
also enable long-term patient monitoring and provide reports to support clinical and research
practices.
ImpediMed – Feb 2018 Page 7 LBL-507-en REV C
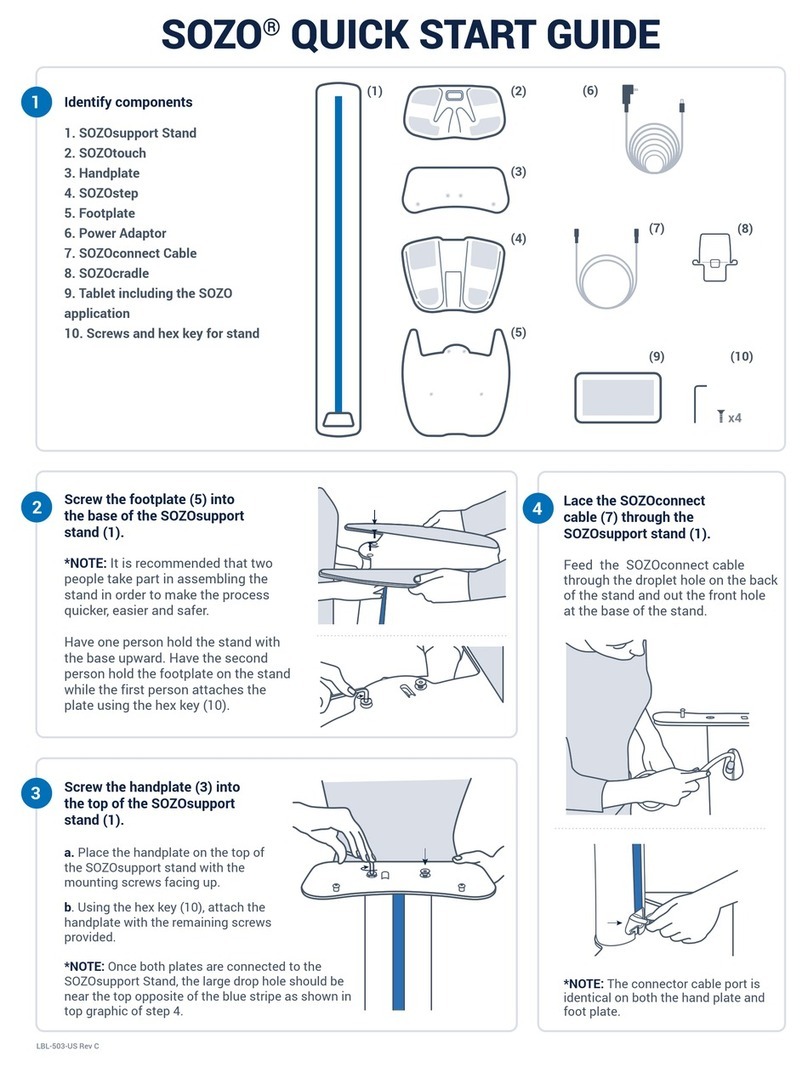
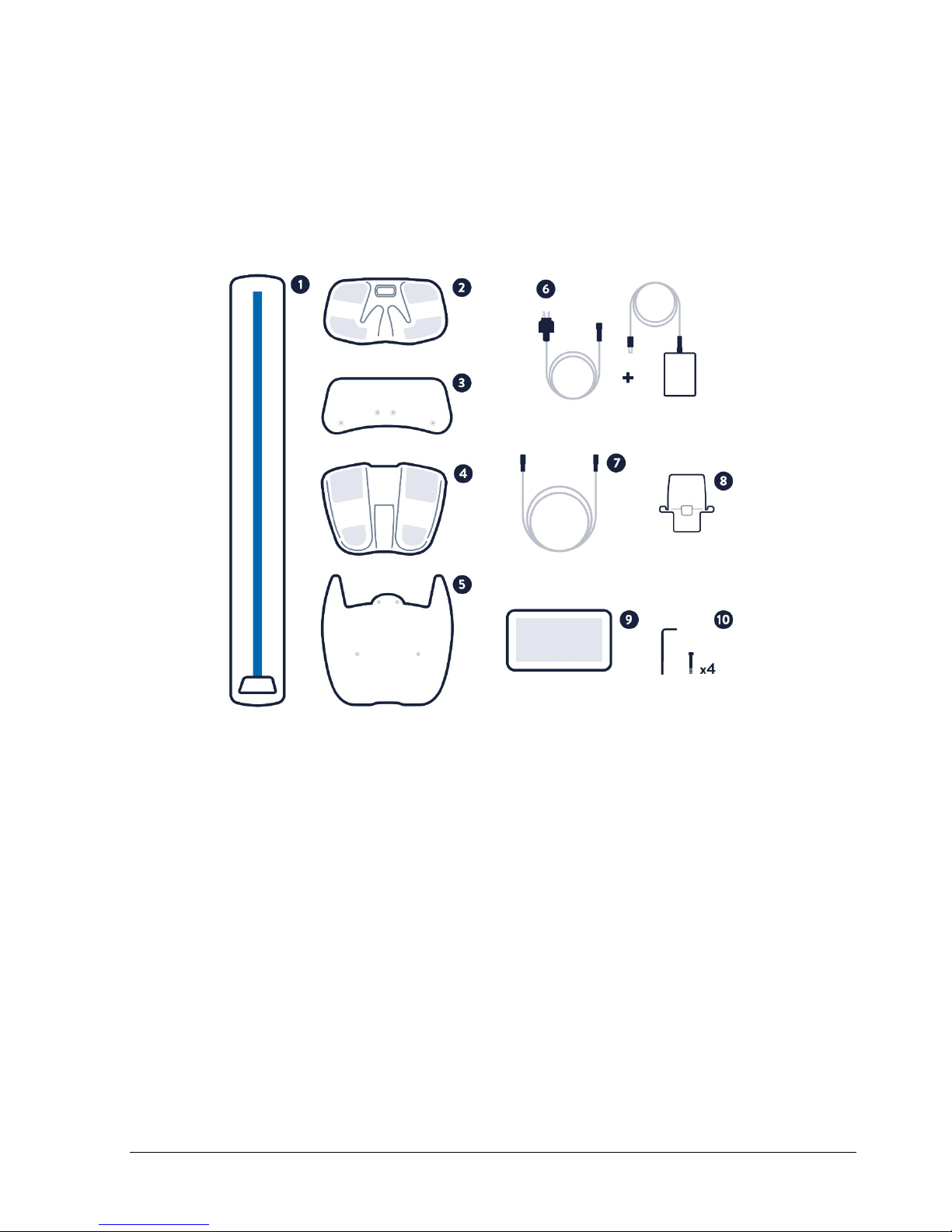
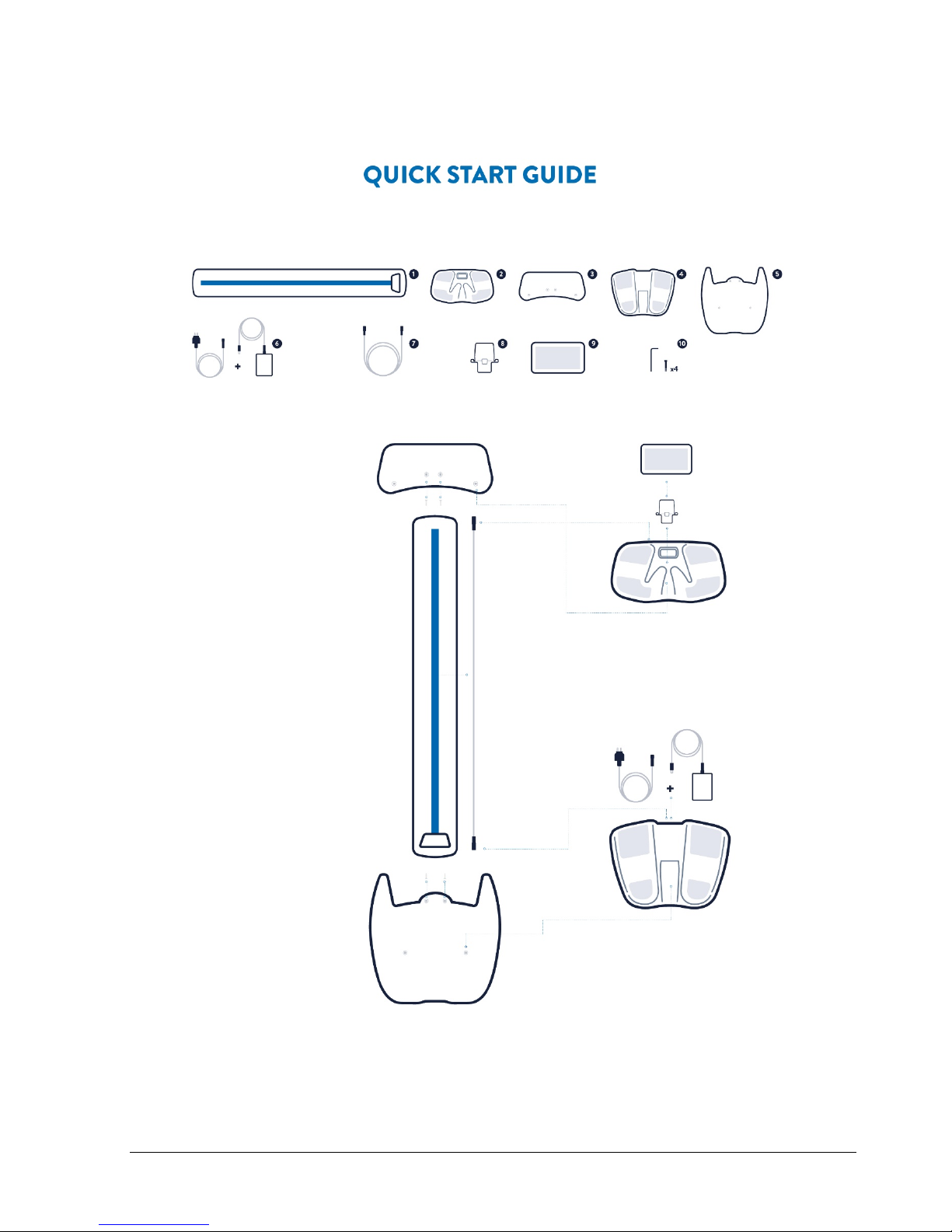
Components of the SOZO System
SOZO by ImpediMed is made up of the following product components and accessories:
•SOZOsupport (1)
•SOZOtouch (2)
•Handplate (3)
•SOZOstep (4)
•Footplate (5)
•Power Adaptor and cord (6)
•SOZOconnect cable (7)
•Tablet holder (8)
•Tablet including the SOZO app (9)
•Screws and hex key for stand (10)
ImpediMed – Feb 2018 Page 8 LBL-507-en REV C
Safety Instructions
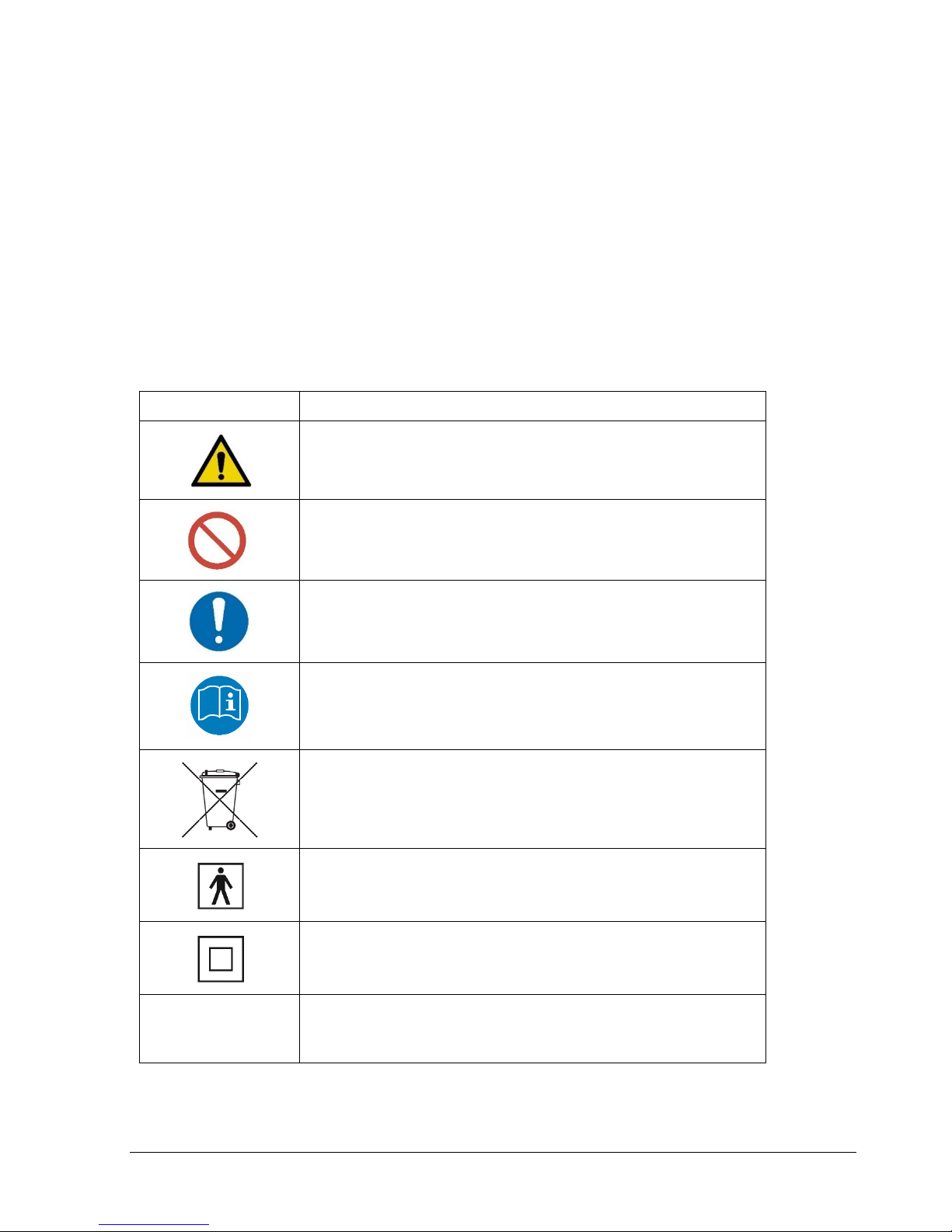
Signs and Symbols
The warning signs and the symbols below are listed in order to use this product safely and
correctly as well as to prevent risk and injury to you and others.
The meanings of the signs are as follows:
Symbol
Definition
Indicates matters in which bodily harm or material damage
or incorrect measurements may arise as a result of
incorrect handling.
What you should NOT do.
An action that must be followed.
Follow instructions for use.
For EU Customers: All products at the end of their life may
be returned to ImpediMed for recycling.
This device is rated BF as per IEC60601-1.
This device meets the standard IEC60601-1-2.
This is a Class 2 medical device.
IP21 Protected from touch by fingers and objects greater than
12 millimeters. Protected from condensation.

ImpediMed – Feb 2018 Page 9 LBL-507-en REV C
Intended Use
SOZO is a medical device, intended for clinical use by operators who have read this manual.
This device is intended for use, under the direction of the operator, for the noninvasive
monitoring and management of fluid levels in patients. This includes use under direction of a
physician in patients with fluid management problems in a variety of medically accepted clinical
applications.
The SOZO app is a software application intended to run on the supplied tablet and provides the
main user interface for the system.
The SOZO device and SOZO app are used in conjunction with SOZOhub, a network-
connected database system that securely stores patient data that is primarily accessed by the
app.
Contraindications
The SOZO device should not be used by:
•Patients with active implanted medical devices, e.g. cardiac
pacemakers, defibrillators or patients connected to electronic life
support devices.
•Patients undergoing external defibrillation.
Warnings
Pregnant Patients:
•While the use of bioimpedance technology in pregnant patients has
been shown to have had no adverse effects, the SOZO device has yet
to be clinically validated for use with that population group.
ImpediMed – Feb 2018 Page 10 LBL-507-en REV C
Precautions
Ensure that you have read and understood this entire instructions for use
document before using the SOZO device. No other specific skill or training is
required to take readings using the device.
Do not allow the SOZO device to come in contact with any liquids.
Only use the power adaptor that is supplied with this device. The use of any
other power adaptor may expose the patient to the risk of electrocution.
Do not use or operate the device in the presence of strong electromagnetic
fields. This Medical Device may interfere with other Medical Devices in its
vicinity.
Devices or other sources can potentially cause interference problems:
•Example 1: heat from a radiant heater.
•Example 2: moisture from a nebuliser.
Keep away from small children or animals. Strangulation due to cables may
occur and small parts may be inhaled or swallowed.
Avoid using on subjects with metal allergies. Allergic reactions may be
caused by the stainless steel used in the electrodes of this device.
Avoid using accessories, detachable parts and materials not described in the
instructions for use, interconnecting this device with other equipment not
described in the instructions for use, or modifying this device in any way.
The use of accessories, transducers, and cables other than those specified
may result in increased Emissions or decreased Immunity of the SOZO
device.
ImpediMed – Feb 2018 Page 11 LBL-507-en REV C
Portable RF communications equipment (including peripherals such as
antenna cables and external antennas) should not be used any closer than
30 cm (12 inches) to any part of the SOZO system, including cables specified
by the manufacturer. Otherwise, degradation of the performance of this
equipment could result.
The effects of degraded sensors and electrodes, or loosened electrodes, can
degrade performance or cause other problems.
Ensure that all data collected from the SOZO device is assessed under
supervision of a physician when managing a chronic disease.
The SOZO device has a maximum weight capacity of 170 kg (375 lbs). Do
not use the SOZO device in a standing position if patient weight exceeds 170
kg (375 lbs).
The SOZO device is intended for indoor use only. Do not use outdoors.
Use and Storage Conditions
Environmental Operating Conditions
The SOZO device must be operated in the following conditions:
•A temperature range of +5°C to +40°C (+41°F to +104°F)
•A relative humidity range of 15% to 93%, non-condensing
•An atmospheric pressure range of 700 hpa to 1060 hpa
The SOZO device has been validated against applicable electrical safety standards for use in
both clinical and home environments.
Environmental Transport and Storage Conditions
The SOZO device must be transported and stored in the following conditions:
ImpediMed – Feb 2018 Page 12 LBL-507-en REV C
•-25°C (-13°F) without relative humidity control and +70°C (158°F) at a relative humidity up
to 93%, non-condensing.
If the unit has been stored at the extremes of these temperature ranges, please allow it to
return to within its operating temperature conditions (approximately 35 minutes) before
installing or using.
Location for Use
Do not place device on any object or material made of metal, other than the
SOZOsupport footplate and handplate.
The SOZO device should not be used adjacent to or stacked with other
equipment and, if adjacent or stacked use is necessary, the SOZO device
should be observed to verify normal operation in the configuration in which it
will be used.
Using the SOZO device on carpet may cause static electricity, which could
damage the equipment. If installing the SOZO device on carpet is
unavoidable, please use the SOZOsupport stand, or an antistatic mat.
Various environmental factors environment may interfere with device
performance including: the effects of lint, dust, light (including direct sunlight),
as well as pets, pests, or children.
Ensure that you have read and understood this entire instructions for use
document before using the SOZO device. No other specific skill or training is
required to take readings using the device.
Surface temperature may exceed 47° C (117° F) in normal use. Do not use
SOZO device if it is hot to the touch. Disconnect the device by unplugging the
power adaptor and call ImpediMed Technical Support.
ImpediMed – Feb 2018 Page 13 LBL-507-en REV C
Setting Up the SOZO Device and Stand
The stand is comprised of the following components 1, 3 and 5 from the component list on
page 8. The screws and hex key (10) are included in the stand packaging.
1. Screw the footplate (5) into the base of the SOZOsupport (1) stand. Note: the footplate
fits to the SOZOsupport end with two cable holes in it.
2. Screw the handplate (3) into the top of the SOZOsupport (1) stand.
3. Lace the SOZOconnect cable (7) through the droplet hole on the back of the stand and
out the bottom hole of the stand.
4. Attach the SOZOtouch (2) to the hand plate by first placing above the screws then
pushing forward to lock into place.
5. Connect the SOZOconnect cable (7) to the SOZOtouch (2).
6. Attach the tablet holder (8) to the SOZOtouch (2) with the ledge of the tablet holder
facing the SOZOtouch sensors.
7. Connect the SOZOstep (4) to the SOZOconnect cable (7), laced through the
SOZOsupport Stand (1).
8. Connect the SOZO power adaptor (6) into the SOZOstep (4) rear plate.
9. Attach the SOZOstep (4) to the footplate (5) by first placing above the screws then
pushing forward to lock into place.
10. Plug the SOZO power adaptor (6) into the nearest wall outlet.
Only use the power adaptor that is supplied with this device. The use of any
other power adaptor may expose the patient to the risk of electrocution.
Ensure that the connector cable is plugged in to both hand and foot plates
before connecting the power supply. If the SOZO device must be moved,
please ensure the power supply is disconnected from the wall before moving
or uninstalling the system.
When plugging the power cord in, the system will automatically run a self-test
to ensure functionality. Do not touch the stainless steel electrodes when a
self-test is running.
For more information on setting up the SOZO system with stand, please visit
www.impedimed.com or contact ImpediMed technical support (contact details on page 43 of
this guide).
ImpediMed – Feb 2018 Page 14 LBL-507-en REV C

ImpediMed – Feb 2018 Page 15 LBL-507-en REV C
Setting Up the SOZO Device without
Stand
Step 1: Connecting SOZOtouch and SOZOstep
Components required:
•SOZOtouch assembly
•SOZOstep assembly
•SOZOconnect cable
Connect SOZOtouch to SOZOstep with the connector cable. (NOTE: the connector cable port
is identical on both the hand plate and foot plate.)
Place the SOZOtouch assembly on a level surface and at a comfortable height to use while
sitting. (e.g. table or desk).
Place the SOZOstep assembly on a level surface beneath/in alignment with SOZOtouch.
Ensure that SOZOstep is positioned in such a way that the power supply can be easily
connected and disconnected.
Step 2: Connecting the Tablet Stand
Components required:
•Tablet
•Tablet stand
•SOZOtouch assembly
Confirm that the SOZOtocuh assembly is stationary and on a level surface. Place the tablet
holder onto SOZOtouch with the ledge of the holder facing the SOZOtouch stainless steel
electrodes. (NOTE: for clinical use, the tablet holder can be reversed with the ledge of the
holder facing away from the electrodes) Place the tablet onto the ledge of the tablet stand.
Step 3: Connecting Power Supply
Components required:
•SOZOstep
ImpediMed – Feb 2018 Page 16 LBL-507-en REV C
•Power supply cable
Locate the power supply port on the back of the SOZOstep assembly and plug the
corresponding end of the power supply cable into that port.
Take the remaining end of the power supply cable and plug it into the nearest wall outlet. The
SOZO device may be safely turned off by unplugging the power adaptor.
Only use the power adaptor that is supplied with this device. The use of any
other power adaptor may expose the patient to the risk of electrocution.
Ensure that the connector cable is plugged in to both SOZOtouch and
SOZOstep assemblies before connecting the power supply. If the SOZO
device must be moved, please ensure the power supply is disconnected from
the wall before moving or uninstalling the system.
For more information on setting up the SOZO system without the SOZOsupport stand, please
visit www.impedimed.com or contact ImpediMed technical support (contact details on page 43
of this guide).
Setup Complete

ImpediMed – Feb 2018 Page 17 LBL-507-en REV C
SOZOhub installation
While setting up the SOZO device, the SOZOhub software will also need to be installed on a
network-connected computer.
SOZOhub is ImpediMed’s SOZO management system and database software that will support
your SOZO use, store patient measurement data, and interface with the SOZO app. Please
refer to the SOZOhub instructions for use on installation and set up.
SOZOhub installation, and creation of at least one user account, must be completed prior to
setting up the SOZO app.

ImpediMed – Feb 2018 Page 18 LBL-507-en REV C
Pairing Tablet and SOZO App to SOZO
System
Step 1
Press the button on the back of the SOZOtouch assembly to enable Bluetoothon the SOZO
device.
Step 2
Locate the Settings section on the tablet and enable Bluetooth. Once Bluetooth is enabled,
select ImpediMed SOZO from the list of available devices. (*Note: Bluetooth must be enabled
first on the SOZO device for the tablet to find it; the SOZO device will be identified by the
SOZO device serial number (SOZO-xxxxxxxxxx) when the tablet scans for nearby Bluetooth-
enabled devices.)
Step 3
Initial setup of SOZO app
The tablet shall come with the ImpediMed SOZO app pre-installed. If the app is not installed,
please contact ImpediMed to walk through the download and installation process.
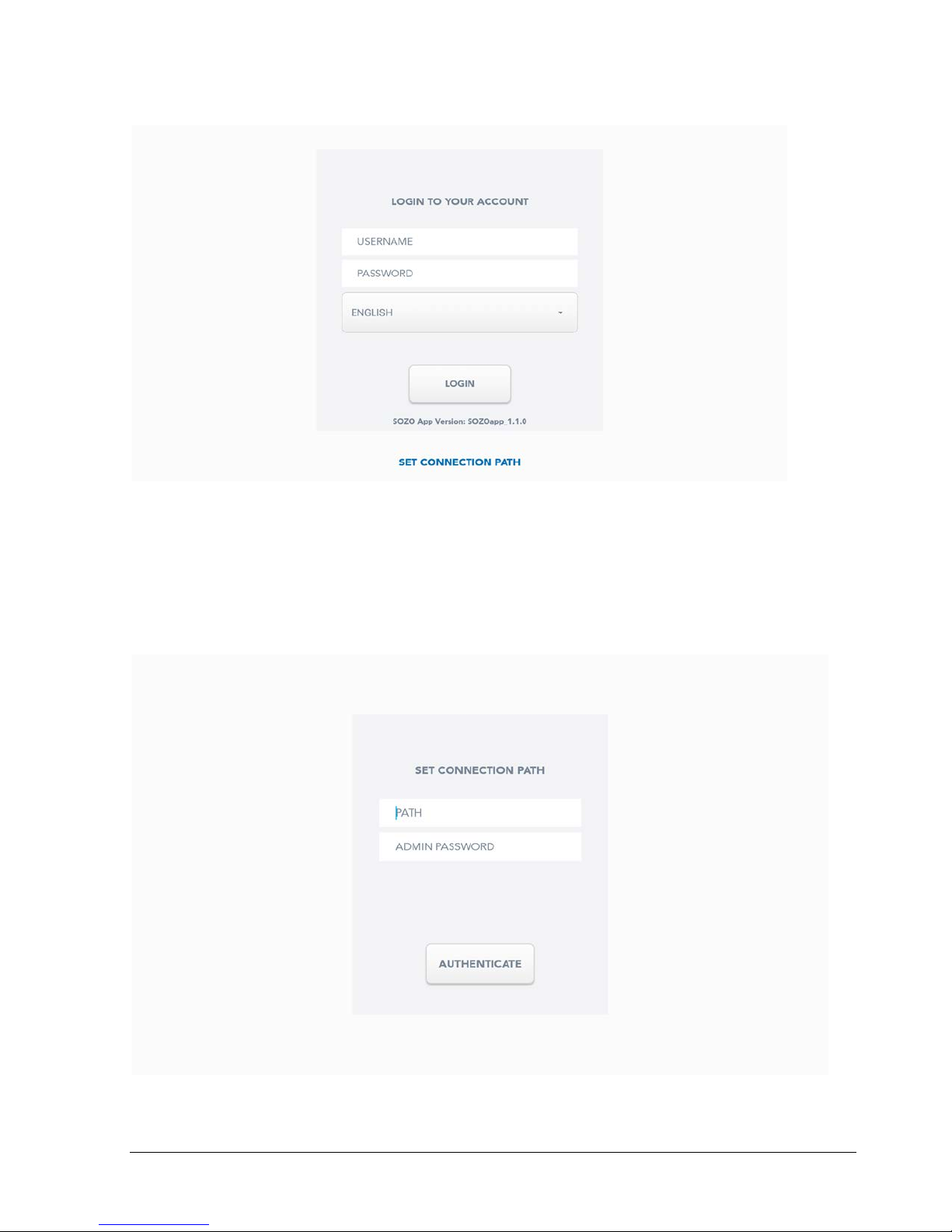
With the SOZO app installed, tap on the icon to start it as you would any other app. This will
bring you to the login screen:
ImpediMed – Feb 2018 Page 19 LBL-507-en REV C
Note that the SOZOapp version may be at a different level from that presented here.
For first-time setup of any SOZO system in the facility, you will need to click on “Set Connection
Path” to link the SOZO app to the SOZOhub database on your hospital server.
ImpediMed – Feb 2018 Page 20 LBL-507-en REV C
The path is a fixed IP address that links to the server directory where the database is stored
and requires authentication before you can proceed.
Clinician accounts are not created through the SOZO app. Accounts are
created through the SOZOhub management system and database. SOZOhub
must first be set up on an accessible network as part of SOZO installation,
and a user account created, to proceed further with set up of your SOZO
system. Please refer to the SOZOhub instructions for use to create new users
to access your SOZO system.
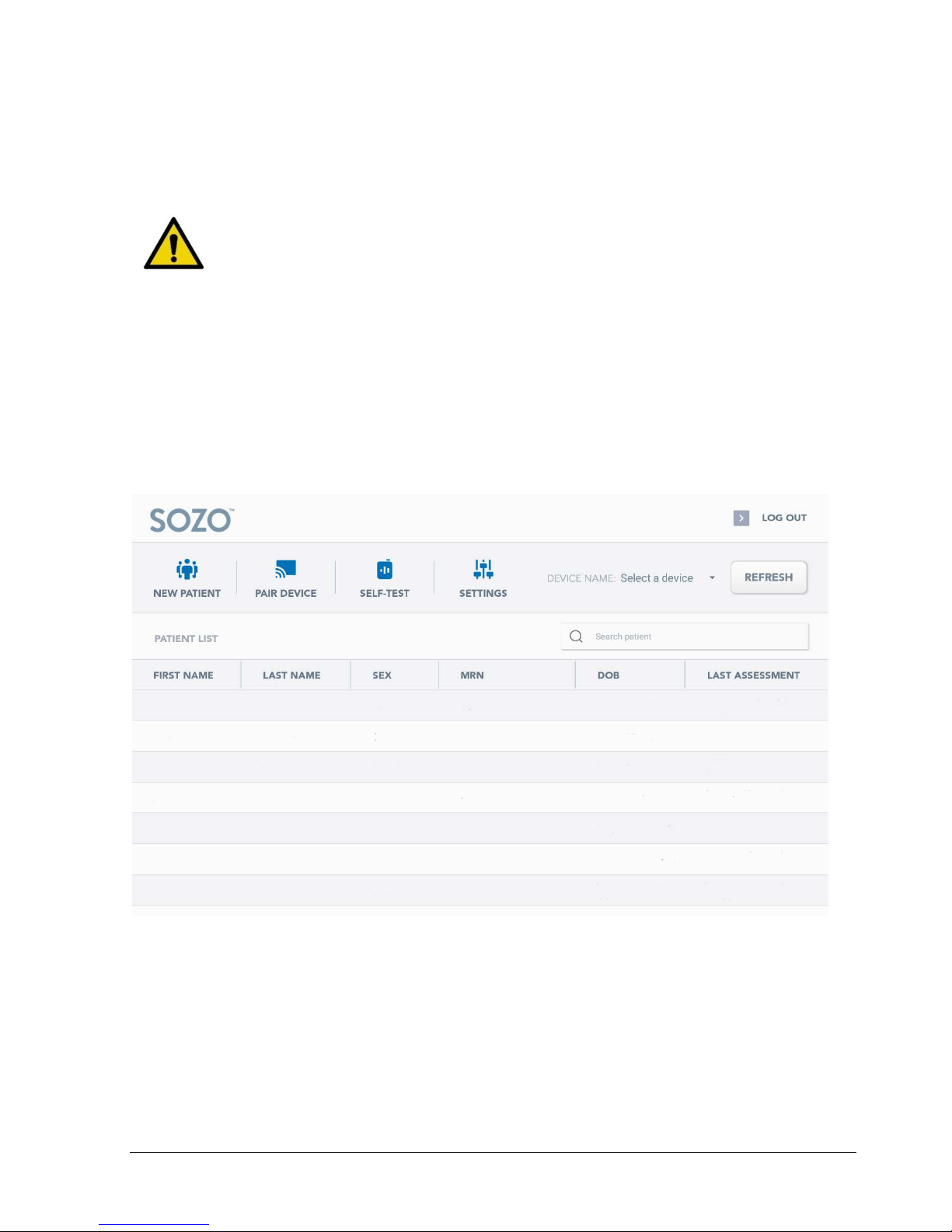
Pairing the SOZO app with the SOZO system
- During first-time setup, the app will need to be paired with the SOZO system. Once the
user account has been created and you have logged in to the dashboard screen, click
on ‘Pair Device’:
The app should then automatically connect with the SOZO device that was paired through
Bluetooth earlier. This can be confirmed by pressing the ‘Settings’ button which will take you to
the overall settings for the device.
Other manuals for SOZO
4
Table of contents
Other ImpediMed Medical Equipment manuals