TNI softFlow 50 User manual

Operating instruction

TNI-System

3
Operating Instruction TNIsoftFlow 50 clinic system
CONTENT
FIRST STEPS
•Theseoperatinginstructionsapplytothe
TNI soft Flow 50
,manufactured2015orlater.
•Pleasereadtheseinstructionsandallwarningscarefully.Otherwise,injuriescouldoccur.
Storethemtobeusableforsubsequentreference.
•Beforeusing
TNI soft Flow 50
forthersttime,thedevicemustbeconguredasinstructedin
TNI soft Flow 50
manual.
•The
TNI soft Flow 50
hastobecleanedanddisinfectedafteruseandchangeofpatient.Pleasenote
operatinginstructions,chapter1.3.7
•Foradditionalinformationandsupport,pleasecontactyourlocalTNImedicalAGcustomerser-vice.
1) DESCRIPTION of TNI softFlow 50 ............................................................... 6
1.1) METHOD.............................................................................................................................................................. 6
1.2) INTENDEDUSE.................................................................................................................................................. 6
1.3) SAFETYNOTES................................................................................................................................................... 6
1.3.1) THISMANUAL................................................................................................................................................... 7
1.3.2) APPROPRIATEAPPLICATION........................................................................................................................ 7
1.3.3) CORRECTUSE.................................................................................................................................................... 7
1.3.4) CORRECTSETUP............................................................................................................................................... 8
1.3.5) ENVIRONMENTALCONDITIONS................................................................................................................ 9
1.3.6) USINGOXYGEN................................................................................................................................................. 10
1.3.7) CLEANING........................................................................................................................................................... 10
1.3.8) FILLINGTHEWATERCHAMBER.................................................................................................................. 11
1.3.9 TRANSPORTOFTHEDEVICE....................................................................................................................... 11
1.3.10)DISPOSAL.....................................................................................................................................................................11
1.4) DESCRIPTIONOFFUNCTION...................................................................................................................... 12
1.5) TRAININGOPTIONS........................................................................................................................................ 12
2) SYSTEM COMPONENTS AND ACCESSORIES.............................................. 13
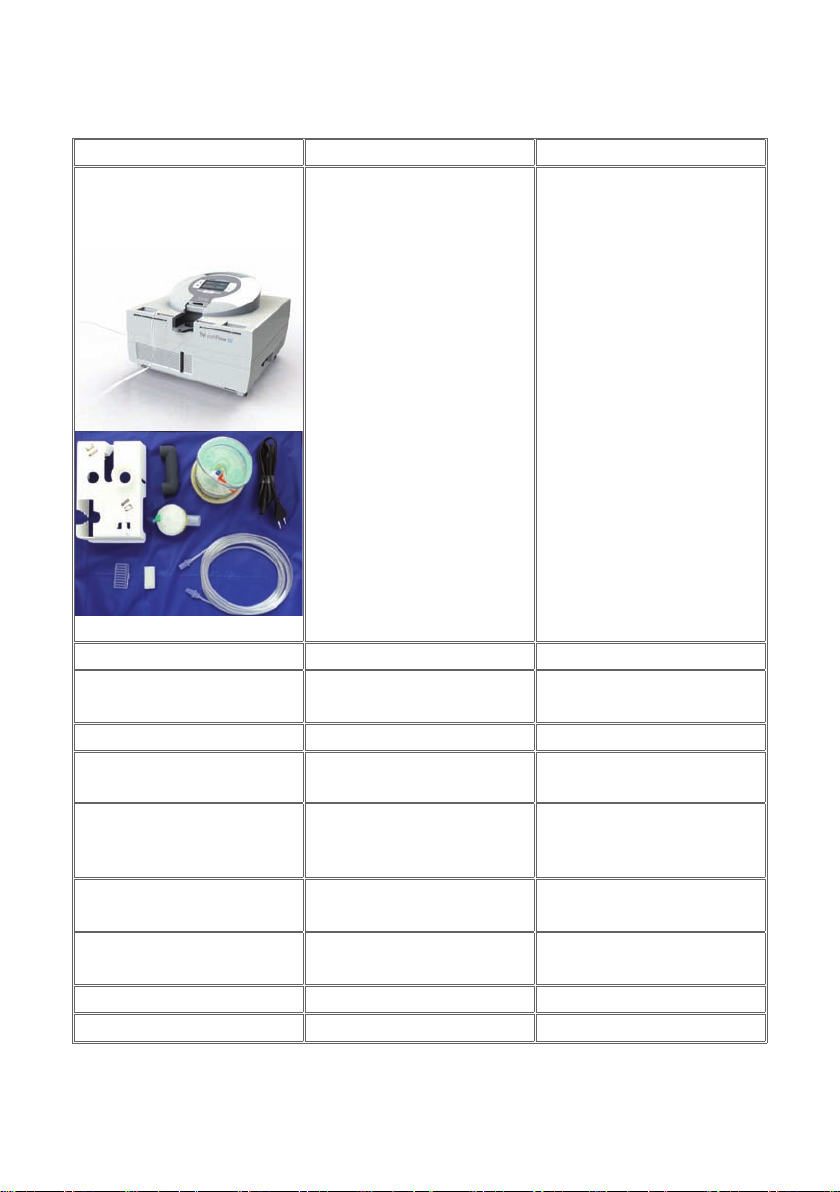
2.1) SCOPEOFSUPPLYofTNI soft Flow 50clinicsystem........................................................................ 13
2.2) HUMIDIFIERHOMECARECOMPLETE....................................................................................................... 14
2.3) ACCESSORIES..................................................................................................................................................... 14
3) STARTUP OF TNI softFlow 50 CLINIC SYSTEM ......................................... 15
3.1) ASSEMBLINGANDCONFIGURINGTHEHUMIDIFIERCLINICCOMPLETE.................................. 15
3.1.1) HUMIDIFIERCLINICCOMPLETE................................................................................................................. 15
3.1.2) CONNECTINGWATERBAG........................................................................................................................... 16

4
Operating Instruction TNIsoftFlow 50 clinic system
3.1.3) FILLINGANINSERTINGTHEHUMIDIFIERHOMECARE..................................................................... 17
3.1.4) INSERTTHEAPPLICATOR............................................................................................................................. 19
3.1.5) REMOVINGAPPLICATOR............................................................................................................................. 19
3.1.6) CONNECTINGOXYGENSUPPLY................................................................................................................ 19
3.1.7) REPLACINGDUSTFILTER............................................................................................................................. 20
3.2) STARTINGANDSTOPPINGTHETNI soft Flow 50............................................................................. 20
3.2.1) SWITCHINGON/OFF.................................................................................................................................... 21
3.2.3) DEACTIVATINGSTANDBY-MODE............................................................................................................... 21
3.3) OPERATINGELEMENTSofTNI soft Flow 50 ..................................................................... 21
3.3.1) OPERATINGELEMENTSofTNI soft Flow 50 ..................................................................... 22
3.3.2) BASICUSERMENUE........................................................................................................................................ 22
3.4) ATTACHINGTHEAPPLICATOR..................................................................................................................... 23
3.5) CHOOSINGTHEAPPLICATOR..................................................................................................................... 24
3.6) STARTINGTHERAPY........................................................................................................................................ 24
3.7) ENDINGTHERAPY............................................................................................................................................ 25
3.8) STARTINGOXYGENSUPPLY......................................................................................................................... 25
3.9) STOPPINGOXYGENSUPPLY........................................................................................................................ 26
4) SELECTING THE THERAPY PARAMETERS AND CONFIGURATION MENU . 26
4.1) THERAPYPARAMETERS................................................................................................................................. 27
4.1.1) STATEMENTONTHEINDICATEDDISPLAYVALUES............................................................................ 27
4.1.2) SETTINGHUMIDITY(DEWPOINT)............................................................................................................ 28
4.1.3) FLOWRATE......................................................................................................................................................... 29
4.2) MENUITEMSWHICHHASBEENAPPROVEDFORSYSTEMCONFIGURATION........................ 30
4.2.1) CHOOSINGTYPEOFHUMIDIFIER............................................................................................................. 30
4.2.2) CHOOSINGNEWPATIENT/CHANGEOFPATIENT............................................................................. 31
4.2.3) SETTINGTHELANGUAGE............................................................................................................................. 31
4.2.4) SETTINGTHETIME........................................................................................................................................... 32
4.2.5) SETTINGTHEDATE.......................................................................................................................................... 33
4.2.6) ALARMVOLUME.............................................................................................................................................. 34
4.3.1) SYSTEMINFORMATION.................................................................................................................................. 34
4.3.2) READINGOUTTHERAPYHOURS............................................................................................................... 35
4.3.3) CLINIC-MENUE................................................................................................................................................. 36
4.3.4) SERVICE-MENUE............................................................................................................................................... 36
5) HYGIENIC MEASURES ...................................................................................... 36
5.1) CLEANING........................................................................................................................................................... 36
5.1.1) CLEANING-ANDCHANGINGCYCLES.................................................................................................... 37
5.1.2) DETERGENTS...................................................................................................................................................... 38
5.1.3) HOUSINGSURFACES...................................................................................................................................... 40

5
Operating Instruction TNIsoftFlow 50 clinic system
Table 1 -
SCOPE OF SUPPLY OF THE CLINIC SYSTEM
..................................................13
Table 2 - FUNCTION KEYS of
TNI soft Flow 50
............................................................22
Table 3 -
SYMBOLS ON THE DEVICE
.............................................................................42
Table 4 - TECHICAL DATA ..............................................................................................43
Table 5 –
FEHLERCODES
.................................................................................................46
Table 6 -
ELECTROMAGNETIC COMPATIBILITY (EMC)
................................................52
TABLES
5.1.4) HUMIDIFIERCLINICCOMPLETE................................................................................................................. 40
5.1.5) HUMIDIFIERHOMECARECOMPLETE;CLINICALUSE....................................................................... 40
5.1.6) DUSTFILTER....................................................................................................................................................... 41
5.1.7)APPLICATORINCLINICALUSE....................................................................................................................... 41
6) DESINFECTION................................................................................................... 41
7) TECHNICAL DATA.............................................................................................. 42
7.1) SYMBOLSONTHEDEVICE........................................................................................................................... 42
7.2) PERFORMANCEPARAMETERS,TECHNICALDATA,DEVICEPARAMETERS................................ 43
7.3) TECHNICALDATA,DEVICEPARAMETERS............................................................................................... 43
8) FEHLERCODES.................................................................................................... 45
9) SERVICE/MAINTENANCE ................................................................................ 50
10) WARRANTY ........................................................................................................ 50
11) DISPOSAL............................................................................................................ 51
12) ELECTROMAGNETIC COMPATIBILITY (EMC) .............................................. 52

6
Operating Instruction TNIsoftFlow 50 clinic system
1) DESCRIPTION of TNI softFlow 50
1.1) METHOD
TNIsoftFlowisasystemmanagingtherapywithnasalinsuationwhichwillbeusedforthetreat-
mentofsleep-relatedbreathingdisordersinclinicalintensivecareandstationarysettingsaswell
asinhomecaresettings.
Aconstant,humidiedandwarmow,whichmayalsocombinedwithoxygen,isappliedtothe
noseviaapplicator(thinnasalcannulawhichactasaninterfacetothepatient.
Therapywithnasalinsuationbasicallyimprovesventilation.
1.2) INTENDEDUSE
Therapywithnasalinsuation(TNI)isintendedforadditionaltreatmentonpatientswithpartialorglobal
respiratoryfailure(insuciency),suchasCOPD(chronicobstructivepulmonarydisorder,ILD(interstitiallung
disease)orsleepapnoeatorelievealsobreathing(respiratory)musculatureandtoimproveventilationas
wellasmucociliaryclearance.
Therapywithnasalinsuationmayonlybecommencedwithamedicalprescription.
Therapywithnasalinsuationisnotintendedforlife-supportpurposes.
Thistherapyisdoneonanindividualbasisaccordingtothemedicaldiagnosis.Itcanbeappliedonadaily
basis(e.g.undersleepingperiod)orsporadicallyifneeded.
TNIsoftFlowmaybeusedonadultsandchildren.Pleasenotethatdierent,specialapplicatorsareavail-
ableforadultsandchildren.
Thepatientshouldbeingoodconditioniftherapyisrealizedathomeandthedeviceisoperatedbythe
patient.Otherwise,theoperationofthedevicehastobeperformedbyaqualiedthirdperson(e.g.nurse).
Thishastobeconsideredespeciallyifthedeviceisusedbyinfantsortoddlers.Forpatientswhicharenot
complianttothedevice,pleasereferto1.3)
TheTNIcanbeplacedonanevenhorizontalsurfaceinhouseandisusedasastationarydevice.Theloca-
tioncanbechangedasneededbutduringtransitnooperationisallowed.
Inclinicalsettingstherespectivecomponentshavetobeexchangedafteruseandchangeofpatient.Please
notepoint5(hygiene).
Inhomecaresettingstherespectivecomponentshavetobecleanedperiodically.
1.3) SAFETYNOTES
• Pleasereadthefollowinginstructionscarefullyastheycontainimportantinformationonthesafe
andresponsiblehandlingofthe
TNI soft Flow 50
.
• Aninitialtraininghastobeperformedbeforethedevicecanbeused
• Followinginstructionsaretheresponsibilityoftheuserofthisunit.
• Anynon-observancewillcausedanger!
• Thedevicecanbeusedinasittingorlyingposition.Itisnotallowedtomovearoundwitharunning
device.Ifaninfantoratoddlerusesthedevice,aqualiedthirdpersonhastoensureproperuse
• Nomodicationofthisequipmentisallowed
• Althoughaselftestofthedeviceisperformedduringstart-up,itisrecommendedtoremoveand
inserttheapplicatorafterstart-uptoverifythecorrectalarmfunctionality.
• Donotusethe
TNI soft Flow 50
ifyoushowallergicreactionswhenincontactwithsilicon

7
Operating Instruction TNIsoftFlow 50 clinic system
1.3.1) THISMANUAL
• Exactadherencetothefollowinginstructionsisaprerequisiteforthesafeandintendedoperation
ofthe
TNI soft Flow 50
anditssuppliedparts.Anynon-observancewillendangeraneective
therapy.
• Withregardtothefundamentalrequirementsofthecurrentlyvaliddirectiveformedicaldevices,the
instructionsforusedescribethepresentstateofthedevice,includingsoftwareandsupplies.
1.3.2) APPROPRIATEAPPLICATION
• The
TNI soft Flow 50
isnotintendedforlife-supportmeasures!
• Atherapymayonlybestartedonprescription.
• Onlyhealthcareprofessionalsmayadjusttheprescribedowrate.
• TheTNIsoftFlowisasystemdesignedfornasalinsuationtherapy.Itcanbeusedclinicallyinin-
patientandintensivecareaswellasinhomecaretoproviderespiratoryassistancetospontaneously
breathingpatients.
• Anasalsupplyofrespiratorygasesleadstoapositiveairwaypressure(PAP)dependingonow
rates.ThishastobeconsideredifthePAPcouldcausethepatienttoshowundesirableresults.The
TNI soft Flow 50
isnotintendedforarticiallife-supportmeasures.
• The
TNI soft Flow 50
isnotsuitablefortreatingacutefailureofrespiratoryfunctions.
• The
TNI soft Flow 50
maynotbeusedforinvasiveventilation.
• The
TNI soft Flow 50
maynotbeusedincaseofcompleteclosureoftheupperairways.
• The
TNI soft Flow 50
maynotbeusedonpatientswhoseairwaysarecircumventedbyabypass.
• Duetohumidicationandamorecomfortableaerialapplication,sideeectssuchasanirritationof
thenasalmucosa,blockednose,and(withpatientssueringfromblood-clottingdisorder)bleedings
ofthenose,arequiteunlikelywiththeuseofthe
TNI soft Flow 50
.Ifsuchsymptomsoccur,the
humidityneedstobesettoahigherlevel(seechapter4.1.2“Setting Humitity”).
• Whennotinuse,the
TNI soft Flow 50
shouldnotbeleftswitchedonforseveralhours.
• Donotusethedeviceifyousuerfromepilepticattacksorveryagitatedsleep
• Donotletchildrenplaywiththehosesorthecablestoeliminatethedangerofstrangulationand
theinhalationorswallowingofsmallparts
1.3.3)CORRECTUSE
• Theunit’shousingmayonlybeopenedbyauthorizedpersonnel.
• Excludedfromthisisequipmentwhichcanberemovedforusageandcleaningpurposes
withoutusingtools.
• Priortoopening,thedevicehasalwaystobeswitchedoandthesystemhastobediscon-
nectedfromthemains.
• IftheTNI soft Flow 50isnotbeingusedoveralongperiodoftime,
• o switchotheTNI soft Flow 50
• o disconnectthepowercordfromthesocket
• o removetheapplicatorand
• o makesurethatthereisnowaterinthehumidicationchambernorinthe
water
container
• Priortoswitchingon,theTNI soft Flow 50alwayshastobecheckedforcorrectassembly.
• Checkallpartsfordamagesanddefects.

8
Operating Instruction TNIsoftFlow 50 clinic system
• Incaseofanyabnormality,switchothepowerswitchanddisconnectthesystemfromthe
powersupplyinordertopreventanydamageorinjuries.
• Whenindoubt,pleasecontactyourlocalTNImedicalAGrepresentative.
• Duringtherapy,theapplicatortubehastoliefreely.Itmaynotbecoveredbyanypillows,
blanketsorclothes.
• Thepatientshouldbecarefulnottointerfereortorestricttheairow.
• Iftheapplicatorisattachedandthepatientisturninginthesamedirectionaroundhis/her
bodyaxisseveraltimes(especiallyduringsleep),pressuremarksmightbecausedandthe
bloodowmightbeimpeded.
• TheavailableUSB-Connectorisforservicepurposesonly
1.3.4) CORRECTSETUP
• PleasemakesurethattheTNI soft Flow 50issetupproperlyanddoesnotshowanydam-
ages.
• PositiontheTNI soft Flow 50correctly:Ithastobeeasytoreachfromthetreatmentarea
andthedisplayshouldalwaysbeeasytoread.
• Ensureanunobstructedairsupply.Theairsupplyaswellastheairpassagemaynotbe
impeded.
• TheTNI soft Flow 50anditsaccessoriescanbeusedwithinpatientsurroundings.Useonly
authorizedaccessoriesmentionedintheinstructionsforuse.
• Useoriginalaccessoriesonly(humidierchamber,applicator-seechapter2.2).Usingthird-
partypartsmayresultinfunctionfailureandhealthhazards.Pleasenotethatinsuchcases
warrantyandliabilityclaimwillexpire.
• Priortoconnectingthedevicetothepowersupplysystem,makesurethattheTNI soft
Flow 50mainsvoltage(110-230V)andmainsfrequency(50-50Hz)correspondtoyour
localcharacteristics.Therequiredinformationisfoundonthedevice’snameplateaswellas
inchapter7,“Technicaldata”.
Connectonlyifalldatacomply!
Usethesuppliedmainscableonly.
• Priortoswitchingon,theTNI soft Flow 50alwaysneedstobecheckedforcompleteness,
correctassemblyandvisibledefects.
• Priortoswitchingon,theTNI soft Flow 50alwaysneedstobecheckedforpropercondi-
tion(completeness,visibledefects,etc.).Forthis,performavisualinspectionofallsingle
partsandcheckthemfordamages.Incaseofabnormalities,thedevicemaynotbe
switchedon.PleasecontactyourlocalTNImedicalAGrepresentative.
• Incaseofdamages,stopusingthedevice.Thisparticularlyappliesifthehousing,plug
connectionandcablesaredamagedaswellasifliquidsgotintothedevice.Insuchcases,
pleasecontactyourlocalTNImedicalAGrepresentativeimmediately.
• Additionalpartswhichneedtobeconnectedtothedevice’sanaloganddigitalinterfaces
havetocomplyveriablywiththeircorrespondingENspecications(e.g.EN60950fordata
processingdevicesandEN60601formedicalelectricaldevices).
• Anybodyconnectingadditionalequipmenttothesignalinputoroutputunitconguresthe
systemandisthereforeresponsiblethatthevalidversionofthesystemcomplieswiththe
systemstandardEN60601-1-1.Foranyqueries,pleasecontactyourlocalTNImedicalAG
representative.
• ThePCconnectionlocatedbelowthecarryinghandleoftheTNI soft Flow 50mayonlybe
usedtoconnectaPC.

9
Operating Instruction TNIsoftFlow 50 clinic system
• Onlytrainedpersonnelorservicetechniciansmayusethesystem.
• ThepatientmaynottouchtheconnectedPCorpartsconnectedtothePC.Theusermay
nottouchthepatientandthePC(orpartsconnectedtothePC)atthesametime.
• ThesensorconnectionlocatedbelowthecarryinghandleoftheTNI soft Flow 50mayonly
beusedtoconnectthe“externaltemperaturemeasuringelement”(itemno.40641018)or
othercomponentsauthorizedbytheTNImedicalAG.Donotconnectunauthorizedcom-
ponentstothissocket!
• Pleasemakesurethatthedeviceispositionedinawaythatthepowerplugcanbediscon-
nectedwithoutdiculties
• AnSD-Cardcanbeusedtostoreinformationindependentlyfromthedevice
1.3.5) ENVIRONMENTALCONDITIONS
• TheTNI soft Flow 50mayonlybeoperatedunderadmissibleambientconditions(see
chapter7,“Technical Data”).Operatingthedeviceunderambientconditionsoutofthe
rangedenedfortheguaranteedperformanceparameterswillresultinreducedperfor-
manceparameters.Iftheambientconditionsareoutofthegivenrange,thedeviceshould
stayino-modeduetosafetyreasons.
Inordertoensureproperuse,itisimportanttolettheTNI soft Flow 50adapttotheambi-
entconditions(roomtemperature).Pleasewaitabout2-4hoursbeforestartingupthede-
vice.ThisappliestotherstusageoftheTNI soft Flow 50andtotransportingthedevice,
i.e.thetransportconditionswereoutofthegivenrangeofambientconditions.Donotuse
thedeviceinhumidandpotentiallyexplosiveroomsorcombustibleatmosphere.
• IntendedfunctioningoftheTNI soft Flow 50maybeimpairedwhenitisoperatedright
nexttoHFelectrosurgerydevices,debrillators,X-rays,transmittedpulses,radiofrequency
interferenceordevicesdesignedforshortwavetherapy.
• DonotusetheTNI soft Flow 50whileperformingthemeasuresmentionedaboveordur-
ingmagneticresonancetomography(MRT,NMR,NMI).
• Activemobilephonesmayonlybeplacednexttothedevicewhenaminimumdistanceof
1misbeingkept.
• Thesystemmustnotbesetupnexttoaheaterandmustnotbeexposedtodirectsunlight
sincethismayinterferewithaproperoperationofthedevice.
• Thesensormeasuringtheambienttemperatureislocatedontherightintheinsideofthe
device.Donotpointanysourceofheat(e.g.heater,...)towardsthissensor.
• Inordertoensureasucientaircirculationaroundthedevice,aminimumdistanceof25
cmtoallsidesistobekept.
• Thedevicemaynotbecovered.
• Toavoidafastaccumulationofdustintheairlter,donotplacetheTNI soft Flow 50 near
theground.
• Thedeviceisintendedtobeusedindoorandshouldbeplacedonanevenhorizontal
surface.
• Toavoiddamage,contaminationormalfunctioning,placethedeviceoutofreachfrom
pets,pestsorchildren
• Operatingthedeviceatalowambientairtemperaturemayresultinaformationofconden-
sateintheapplicator.Keeptheboxedapplicatorawayfromdirectsunlightandstoreitina
dryplace.

10
Operating Instruction TNIsoftFlow 50 clinic system
1.3.6) USINGOXYGEN
• DonotputtheapplicatorontheTNI soft Flow 50oranyotherelectricallydrivendevice.
• TheTNI soft Flow 50mayonlybeoperatedusingtheprovidedconnectionunits.Itisnot
allowedtooperatethedeviceusingotherconnectionunits.
• Pleasemakesuretoreadtheusermanualofyouroxygensourcecarefully.Ifthereareany
openquestionsconcerningusageorconnectionofthesource,pleasecontactyouroxygen
vendororourhotline
• Mountyouroxygensourcecorrectly,especiallyifit’sanoxygenbottle,topreventdamage.
Pleaserefertotheusermanualoftheoxygensource.
• Oxygenvalvesmaynotcomeintocontactwithoil,greaseoranyammableliquids.Dueto
theriskofre,smokingandopenrearestrictlyprohibited!
• Thedevicemaynotbeoperatedinclosedareasproducingorusinganaestheticsand/or
nitrousoxide.
• Especiallywhenaddingclinicaloxygen,thefollowingsafetyguidelinesneedtobeob-
served:
oDonotplacetheTNI soft Flow 50directlyontheoor.Keepaminimum
distanceof40cm.
oKeepaminimumdistanceof40cmtothewall.
oKeepaminimumdistanceof80cmtootherelectricaldevices.
• Priortoswitchingon,alwaysmakesurethattheconnectionunit(s)is/areconnectedprop-
erlytotheintendedgassamplingpoint(s).
• Ensureasafeandsolidconnection.
• Oxygensupportscombustionprocesses.Whenusingoxygenduringtherapy,smokingand
openrearestrictlyforbidden.
• Oxygenvalvesneedtobekeptfreeofgrease.
• Improperconnectionoftheexternaloxygensourcemayresultinaninsucienttherapy.
• Cautionwhenhandlingoxygen!Riskofre!
• Severaldeathsoccurredinhospitalsinthepastduetopatientswhosmokeddespitebeing
treatedwithoxygen.Inordertotakeadrag,theoxygentubewasremovedfromtheface
andputonthebed.Thismadeitpossiblefortheoxygentospreadacrossthebeddings
andthepatient’sclothes.Thepatientfellasleepandthelitcigarettesetthebeddingonre.
• Itwasnotpossibletoghtthereduetothespreadoxygen.Itkeptburningevenafterthe
attemptstoextinguishit.Thepatientnallydiedoftheburnings
1.3.7) CLEANING
• Thedevicemayonlybecleanedwhenbeingcompletelydisconnectedfromthepower
supplysystem.Priortoanycleaningmeasure,switchothemainswitchlocatedattheright
sideofthedeviceandunplugthepowercordfromthedevice’sfemaleconnector.
• Pleasenotechapter6,“Hygiene”.Notobservingtheinstructionsoncleaninganddisinfec-
tioncanleadtoabacterialcontaminationandmayendangerthepatient!Overdosing
disinfectantscancausematerialdamages.Avoidcalcication(seechapter6,“Hygiene”).
• Pleasenotethereplacementcyclesofaccessoriesanddisposables(seechapter6,“Hy-
giene”).Whenexceedingthesetimeperiodsornotreplacingtheseitems,properusecan
nolongerbeensured.
• Duetobiocompatibilityreasons,theapplicatormaynotcontinuouslybewornforlonger
than24hours.Replacetheapplicatorevery24hoursandnotethereplacementcycles.

11
Operating Instruction TNIsoftFlow 50 clinic system
• TheTNI soft Flow 50isalwaystobecleanedanddisinfectedpriortothevisitofanew
patient(formoredetailedinformation,seechapter6,“Hygiene”).Theapplicator,MRPlter,
airliftandhumidierchamberneedtobereplaced.
• Duetohygienicreasons,notmorethanonepatientmayusethesameapplicator.
1.3.8) FILLINGTHEWATERCHAMBER
• Whenopeningthedeviceimmediatelyafterturningito,pleasenotethattheinnerparts
ofthedevice(metallicbottomofthewatercontainer,heatingplate)mightbehotandmay
thereforenotbetouched.Pleasewaitacoupleofminutesuntilthedevicehascooleddown.
• Undernocircumstancesmustuidsgetintothedevice!
• AlwaystakethewatercontaineroutoftheTNI soft Flow 50tollitup.Marksonthestor-
agecontainerindicateminimumandmaximumllinglevel.Fillupthecontainerwithinthis
area,maximumuptothemarklabeled“max”.
• Thewatercontainermayonlybelledwithdrinkingwater.Donotuseadditives!(seechap-
ter3.1.1Humidiercliniccomplete,und3.1.3.Fillingandinsertingthehumidierhome-
care).Ifthedeviceislledwithnon-recommendedadditives,thepatient’sairwaysmaybe
impaired!
• AssoonasthehumidierislledupwithwaterandinsertedintheTNI soft Flow 50again,
theTNI soft Flow 50shouldnotbemovedquickly,beextremelyinclinednortransported.
Withsuchactivities,watermightuncontrollablygetoutofthehumidierintothedevice
whichmayimpaircorrectfunctioningofthedevice.
• Removethehumidierbeforetransportingthedeviceorchangingitsposition.
1.3.9 TRANSPORTOFTHEDEVICE
• Inordertoavoiddamages,becarefulwhentransportingorstoringthedevice!
• ThewaterchambermaynotcontainwaterduringtransportationoftheTNI soft Flow 50.
• Thedevicemayonlybetransportedinanuprightandintendedposition.
• Donotdropthedevicesincethismaycausehousingdamagesandimpairproper
functioning.
• Incaseofdroppingthedevicenonetheless,makesurethattheTNIisinpropercondition
(completeness,novisibledefects,etc.).Inordertoensurethis,alwaysperformavisual
checkfordamagesofthesinglepartspriortostartingup.Incaseofabnormalities,immedi-
atelycontactyourlocalTNImedicalAGrepresentative.
• Ifyouaretransportingtheexternaloxygensource,pleaseconsulttheusermanualofthat
devicefortransportinstructions.
1.3.10)DISPOSAL
InaccordancewiththeGermanElectricalandElectronicEquipmentAct,themanufactureris
responsibleforthedisposaloftheTNI soft Flow 50(formoreinformationondisposal,seechapter
11).Todisposeoftheunit,contact:
TNImedicalAG Telephone:+4993120792902
Hofmannstraße8 Telefax:+4993179292918
www.tni-medical.de

12
Operating Instruction TNIsoftFlow 50 clinic system
1.4) DESCRIPTIONOFFUNCTION
TNIsoftFlowisasystemmanagingtherapywithnasalinsuation:
Aconstant,humidiedandwarmow,whichmayalsocombinedwithoxygen,isappliedinthe
noseviaapplicator(thinnasalcannulawhichactasaninterfacetothepatient).Technically,it
consistsofaventilationunitandahumidierunit.
Theblowerabsorbsair,compressesitandforwardsitviahumidier.Here,theairowsover
heatedwater.Theheatedandhumidiedwaterthusobtainedachievesdewpointsfrom30-37°TP
(canbesetindividually).
2humidicationmodelsareprovided;bothcanbeusedindividually,“Humidiercliniccomplete”
and“Humidierhomecarecomplete”.
Theheatedandhumidiedair/air-oxygenmixtureowsinthenoseandupperrespiratorytract
viaapplicator.
Theairoutowattheapplicatorinthenosesimulatesnasalcannulausedinoxygentherapy.
Particularemphasiswasgiventoahighwearingcomfortandlownoiselevel.
WewishyouagoodandrecreativetimewiththeTNI soft Flow 50
1.5) TRAININGOPTIONS
AninitialtrainingisperformedbyTNIoranauthorizedpartnerbeforethedeviceisused.
Formoretrainingoptions(asanin-depthservicetraining)orotherinformation,pleasecontact
yourlocalTNImedicalAGcustomerservice.
13
Operating Instruction TNIsoftFlow 50 clinic system
2) SYSTEM COMPONENTS AND ACCESSORIES
2.1) SCOPEOFSUPPLYofTNI soft Flow 50clinicsystem
Scopeofdelivery ArticleNo.
TNI soft Flow 50clinic-System
english
40610021
Humidiercliniccomplete Set 40620100
PowerconnectingcableTNI
soft Flow 50,typeC,1,8m
1Unit 40641150
Dustlterreserves 5Unit 40620060
Oxygentube,4m 1Unit 40641112
Operatinginstructions
TNI soft Flow 50clinicsystem
1Unit 30221041
ShortmanualTNI soft Flow
50clinicsystem
1Unit 30221061
OrderformAccessoriesTNI
soft Flow 50international
1Unit 30222201
Declaration 1Unit 30222220
SDcard4GBTNI soft Flow 50 1Unit 40641103
Table 1 - Scope of Delivery of the clinic system

14
Operating Instruction TNIsoftFlow 50 clinic system
2.2) HUMIDIFIERHOMECARECOMPLETE
ThestandardpackageofTNI soft Flow 50clinicsystemincludesthe“humidierclinicsystem
complete”(Art.No.40620100).
Thesystemmaybeusedalternativelywiththe“humidierhomecarecomplete”.(Part-No.
40620000)
Detailsconcerningcorrectusepleasenotechapter3.1.3.
WARNING
USE OF THE “HOMECARE HUMIDIFIER” IN EVERYDAY HOSPITAL ROUTINES
When using this type of humidier, there is no MRP lter integrated. If the patient is likely to further
use the system in his/her home environment, this type of application is recommended to be used
in hospital. The patient will become acquainted with the system and the healthcare professionals
can tell a safe continuation of the following homecare therapy. When being reused in hospital, the
system is to be disinfected thoroughly before being used on a new patient.
SAFETY NOTE
Please note the hygiene measures (chapter 6).
2.3) ACCESSORIES
Accessories,sparepartsandacurrentlistofapplicatorsforrespectiveTNI soft Flow 50systems
areavailableatTNImedicalAG.
Orderform Articlenumber 30222201
Productcatalogue Articlenumber 30222231
Forfurtherinformationpleasenote www.tni-medical.de

15
Operating Instruction TNIsoftFlow 50 clinic system
3) STARTUP OF TNI soft Flow 50 CLINIC SYSTEM
3.1) ASSEMBLINGANDCONFIGURINGTHEHUMIDIFIERCLINICCOMPLETE
• Placetheunit/systemhorizontallyonaplanesurface.
• Makesurethatthesystemislocatedbelowheadheight.
• Insertthemainscableintothepowersocketattheright
sideofthedevice.
• theninsertthepowerplugintothepoweroutlet.
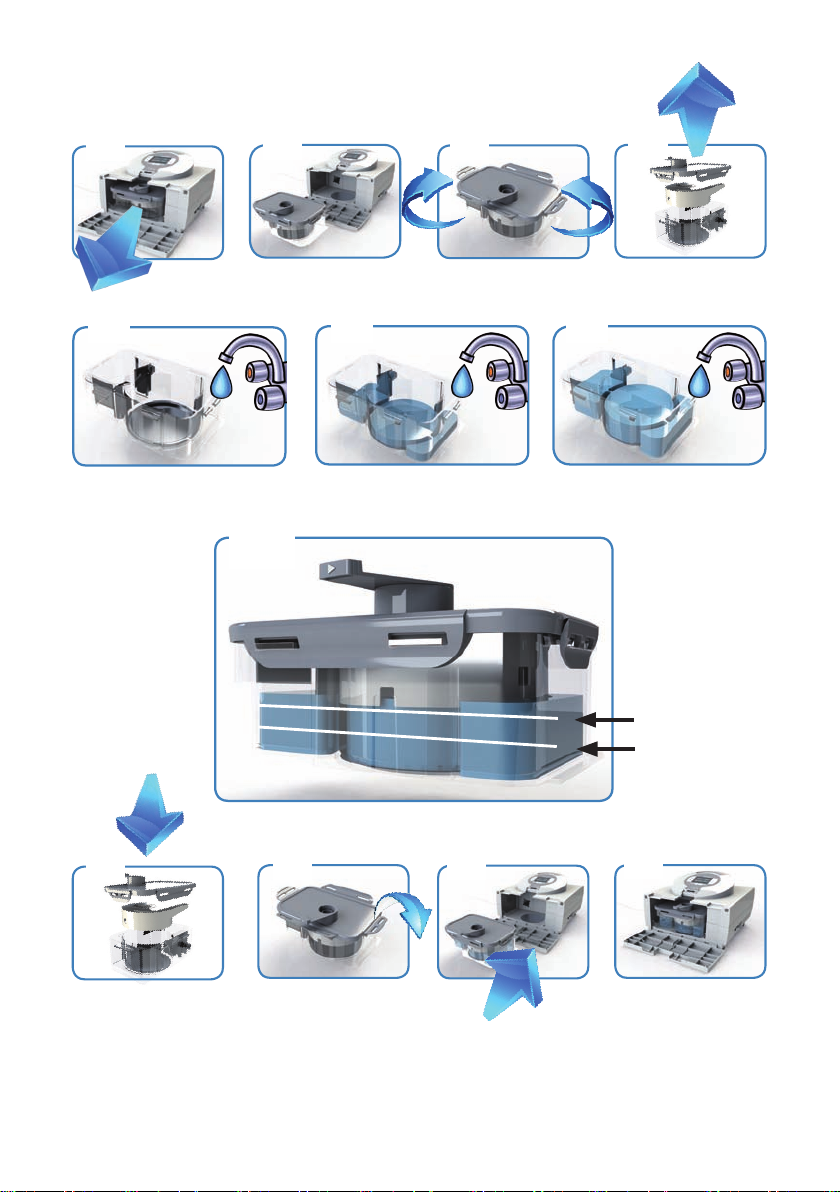
3.1.1) HUMIDIFIERCLINICCOMPLETE
• Thehumidiercliniccompletecontains:
o Humidierrackclinic
o Clear-Guard3bacterialter;MRPhygieniclter
o Humidierchamberauto-Fill
o Airlift
• Removethehumidierrackclinicandthehumidierclinic-
hygienicsetfromthepackagingandassemblethemaccordingto
theoperatinginstructions.
• Pleasereferadditionallytothedescriptiveimagesequence.
• Pushthehumidiercliniccompletefromfrontintothedeviceand
closethefrontofthehousing.
• Fixasterilewaterbagtotherespectivehoseoftheauto-ll
chamber.
WARNING
Hygiene
• Use authorized parts only.
Make sure that the hygiene regulations are met.
• Use originally packed and unexpired parts only.
• Do not apply already used disposables, e.g. humidier chamber,
MRP lter, air lift, etc.
• Use sterile water in clinical treatment.
SAFETY NOTE
In order to ensure an optimal treatment,
• Do not use the humidier chamber autoll after it had been
dropped or run dry, which will trigger the alarm “Rell water”.
• Remove the clinical humidier completely BEFORE transporting,
tilting or moving the device.

16
Operating Instruction TNIsoftFlow 50 clinic system
3.1.2) CONNECTINGWATERBAG
• Attachthesterilewaterbagtothehang-
ingbracketapp.1mabovetheunit,and
pushthebagspikeintothettingatthe
bottomofthebag.Opentheventcapon
thesideofthebagspike.Thechamber
willnowautomaticallylltotherequired
levelandmaintainthatleveluntilthe
waterbagisempty.
• Toensurecontinualhumidication,make
surethatthewaterchamberand/orwaterbagisalwayslledwithwater.
• Checkthatwaterowsintothechamberandismaintainedbelowthellline.Ifthewater
levelrisesabovethellline,replacethechamberimmediately.
12
4 5 6
78 9 10
11 12 13
1 2 3

17
Operating Instruction TNIsoftFlow 50 clinic system
3.1.3) FILLINGANINSERTINGTHEHUMIDIFIERHOMECARE
• TheHumidierhomecarecompletecontains:
o Waterchamber
o Cycloneelement
o Lid
• RemovethepreassembledhumidierhomecarecompletefromthepackagingorfromTNI
soft Flow 50
• Pleasereferadditionallytothedescriptiveimagesequence
• Removethelidappingthelockingtabupwardsonallsides
• Removethecycloneelement(pullitoutupwards)
• Fillthewaterreservoirwiththerecommendedwateruptothe“max”mark
o Afterllingthewaterlevelmustbeintheareaof„min/max“
o Themark„max“mustnotbeexceeded
• Insertthecycloneelement.
• Closethelidandlockit.
• Carefullypushthehumidierrackhomecarecompleteintothedevice
• Makesurethatnowatercanaccessintothesystem
• Closethehousingfrontlid.
WARNING
Hygiene
• Use authorized parts only.
• Make sure that the hygiene regulations are met.
• Use originally packed and unexpired parts only.
• Do not apply already used disposables, e.g. humidier chamber, MRP lter, air lift, etc.
• Use sterile water in clinical treatment
SAFETY NOTE
In order to ensure an optimal treatment,
• Do not use the humidier chamber autoll after it had been dropped or run dry, which will
trigger the alarm “Rell water”.
• Remove the clinical humidier completely BEFORE transporting, tilting or moving the device.
18
Operating Instruction TNIsoftFlow 50 clinic system
3
567
124
910 11 12
8
max.
min.

19
Operating Instruction TNIsoftFlow 50 clinic system
3.1.4) INSERTTHEAPPLICATOR
• Choosetherightapplicator.
• Removeitfromthepackagingandinserttheapplicatorinto
thedesignatedopeningofthedevice.
Inserttheapplicatorplugfromaboveintotheopeningand
pressdownwithlessforceuntilitsnapsin.
• Thelockinglevermovestotheleftstop.
WARNING
In order to avoid burnings,
• Do not modify the applicator in any way
• Make sure that the applicator is not heated up to more than
room temperature (e.g. by means of a blanket, electric re...)
since this may cause serious injury.
• Do not use insulating sleeves or accessories that have not been
authorized or recommended by TNI medical AG.
SAFETY NOTE
• In order to minimize interference with the supervised signal, position the heated applicator
tube away from any electronic monitoring electrode (EEG, ECG, EMG, etc.).
3.1.5) REMOVINGAPPLICATOR
WARNING
In order to prevent mechanical destruction,
• Remove the applicator from the retainer without eort.
• Do not use force if it is not possible to remove the applicator right away.
• Do not use any tools to remove the applicator.
SAFETY NOTE
• Do not let any foreign substances or objects get into the system’s
applicator opening.
• Movethelockingleverundertheapplicatortotheright
• Theapplicatorisreleasedfromitslocking
• Removetheapplicatorplugupwardsfromtheholder
• Now,thesystemcanbeusedwithanotherapplicator.
3.1.6) CONNECTINGOXYGENSUPPLY
WARNING
In order to prevent burnings,
• do not modify the applicator in any way.
• note the safety guidelines in chapter 1.3.6.
• do not place the TNI soft Flow 50 device on the oor. Keep a minimum distance of 40 cm!
• keep the minimum distance of 40 cm to the wall.
• keep the minimum distance of 80 cm to other electric devices.
• note that an external oxygen source not being completely connected may lead to an in-
adequate therapy.
• Be very careful when handling oxygen! Fire hazard!

20
Operating Instruction TNIsoftFlow 50 clinic system
SAFETY NOTE
• Oxygen supports combustion process. Smoking and open re are not permitted when oxygen
is used during therapy.
• Several deaths occurred in hospitals in the past due to patients who smoked despite being
treated with oxygen. In order to take a drag, the oxygen tube was removed from the face and
put on the bed. This made it possible for the oxygen to spread across the beddings and the
patient’s clothes. The patient fell asleep and the lit cigarette set the bedding on re. It was
not possible to ght the re due to the spread oxygen. It kept burning even after the attempts
to extinguish it. The patient nally died of the burnings.
• Theconnectiontotheoxygensourceislocatedontheleftsideofthedevice.
• ConnecttheTNI soft Flow 50withtheoxygensupplyviatheoxygentubesupplied.
• DonotsupplyoxygenbeforetheTNI soft Flow 50hasstarted.
• Therequiredrateofoxygenwillbeset/adjustedattheoxygensupply.
3.1.7) REPLACINGDUSTFILTER
• Pleasechangethedustlteratregularintervals(atleastevery3month)
• Takethedustltercoveroutoftheholder(atthebackside)
• Alightdownwardpressuretotheapreleasesthelock
• Thedustltercovercanberemoveddownwards.Removethedustlter.
•
• Insertanewdustlterincaseofachange.
• Insertthedustltercoverfrombelowandlockitwithlightpressureontotheupperpart.
• Protectthelterfromdirectsunlightandhumidity.
3.2) STARTINGANDSTOPPINGTHETNI soft Flow 50
WARNING
To avoid electric shocks,
• Make sure that the TNI soft Flow 50 is dry before connecting it to the power supply.
• Make sure that the voltage (110-230 V) and mains frequency (50-60 Hz) admissible for the
TNI soft Flow 50 comply with your local characteristics.
Other manuals for softFlow 50
4
Table of contents
Other TNI Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual