IFU 12392 Rev B Invuity Customer Service: +1-866-711-7768 4
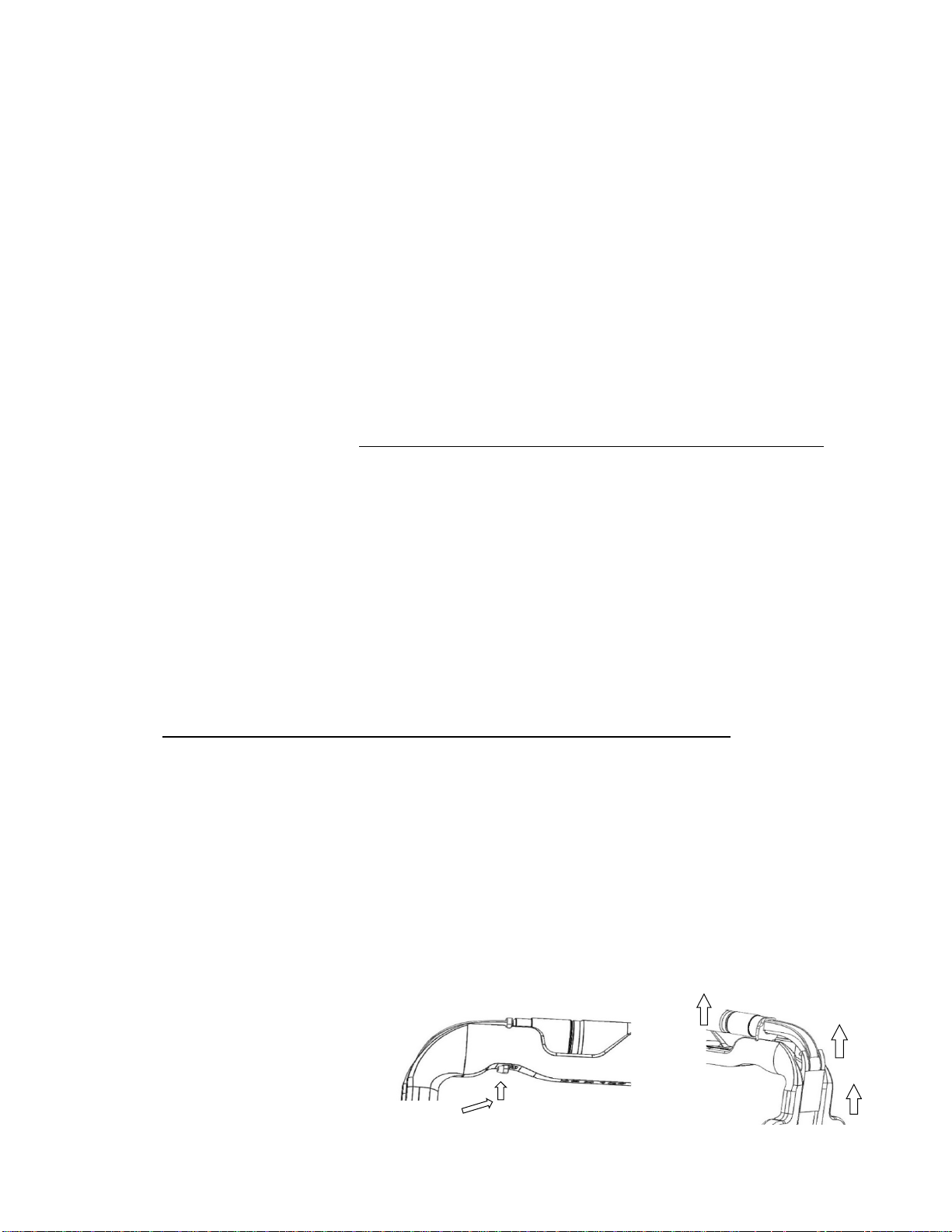
13. Do not pry the PhotonGuide from the Retractor to remove. Prying or pulling from the bottom of the PhotonGuide can
cause damage to the device (Figure 2).
14. Upon completion of the procedure, turn the surgical light source off, disconnect the Fiber Optic Cable from the
PhotonGuide. Remove the PhotonGuide from the retractor.
15. Disconnect the Fiber Optic Cable from the surgical light source. When removing the Fiber Optic Cable from the light
source, grasp the connector, not the black insulation, as pulling on the insulation with excess force can cause product
damage.
16. Discard the PhotonGuide(s) according to hospital procedures and in accordance with local, state and federal laws
and regulations.
Note: Refer to the Instructions for Use for the Invuity Fiber Optic Cables, Sterilization Trays and Retractors
and Accessories for additional information.
Warranty
INVUITY warrants, for the earlier of (i) the expiration date, if applicable, or (ii) one (1) year from the date of purchase, that
this medical device is free from defects in both material and workmanship when used normally for its intended purposes.
Suitability of the medical device for any surgical procedure shall be determined by the user alone (in light of relevant
instructions for use) and not by INVUITY, and such user shall be responsible for understanding how to use the device for
its intended purpose. In the event that purchaser believes that any INVUITY medical device is defective, purchaser will
promptly notify INVUITY. Any INVUITY brand named instrument delivered from INVUITY proving to be defective will, as
purchaser’s sole and exclusive remedy, be replaced at no charge or the amount paid refunded, at INVUITY’s discretion,
so long as the defect is discovered and reported to INVUITY within the earlier of thirty (30) days of discovery or one (1)
year of purchase, and INVUITY determines that the product is defective and is covered by the warranty. Notwithstanding,
INVUITY shall not be responsible for normal wear and tear, including minor cosmetic damage compromise or defect
caused by re-sterilization. INVUITY does not repair, sharpen, recoat or otherwise refurbish any used product. The
foregoing limited warranty and limited obligation of replacement is void and of no effect in the event that there is any
modification made to the medical device or the medical device is used for any purpose other than its intended purpose.
LIMITATION OF LIABILITY. INVUITY DOES NOT ACCEPT LIABILITY TO CUSTOMER OR ANY THIRD PARTY
BEYOND THE REMEDIES EXPRESSLY SET FORTH HEREIN, INCLUDING ANY LIABILITY FOR PRODUCTS NOT
BEING AVAILABLE FOR USE. IN NO EVENT SHALL INVUITY BE LIABLE FOR LOST PROFITS, LOSS OF BUSINESS,
LOSS OF USE, COSTS OF PROCUREMENT OF SUBSTITUTE GOODS, OR ANY OTHER CONSEQUENTIAL,
EXEMPLARY, INCIDENTAL, SPECIAL, INDIRECT OR PUNITIVE DAMAGES, HOWEVER CAUSED AND UNDER ANY
THEORY OF LIABILITY, WHETHER BASED IN CONTRACT, TORT (INCLUDING NEGLIGENCE) OR OTHERWISE
EVEN IF ADVISED OF THE POSSIBILITY OF SUCH DAMAGES, OR FOR ANY CLAIM BY ANY THIRD PARTY, EXCEPT
AS EXPRESSLY PROVIDED HEREIN.
Customer must obtain a Return Material Authorization (RMA) to return product to Invuity. Returned product must be
thoroughly cleaned and sterilized.
CUSTOMER ACKNOWLEDGES UNDERSTANDING THAT NO OTHER REPRESENTATION OR WARRANTY OF ANY
KIND, EXPRESS OR IMPLIED, WITH RESPECT TO THE PRODUCT AS TO ITS MERCHANTABILITY, FITNESS FOR
A PARTICULAR PURPOSE OR ANY OTHER MATTER, EVEN WHEN APPLIED OR UTILIZED IN ACCORDANCEWITH
ITS INSTRUCTIONS, ARE MADE OR GIVEN BY INVUITY. AND INVUITY EXPRESSLY DISCLAIMS ALL SUCH
REPRESENTATIONS AND WARRANTIES.
Invuity, the Invuity logo, and PhotonGuide are registered trademarks of Invuity, Inc. The products referenced in this
document may be covered by one or more patents. See: www.invuity.com/patents. All rights reserved.
Figure 2: Do not pry the PhotonGuide from
the Retractor