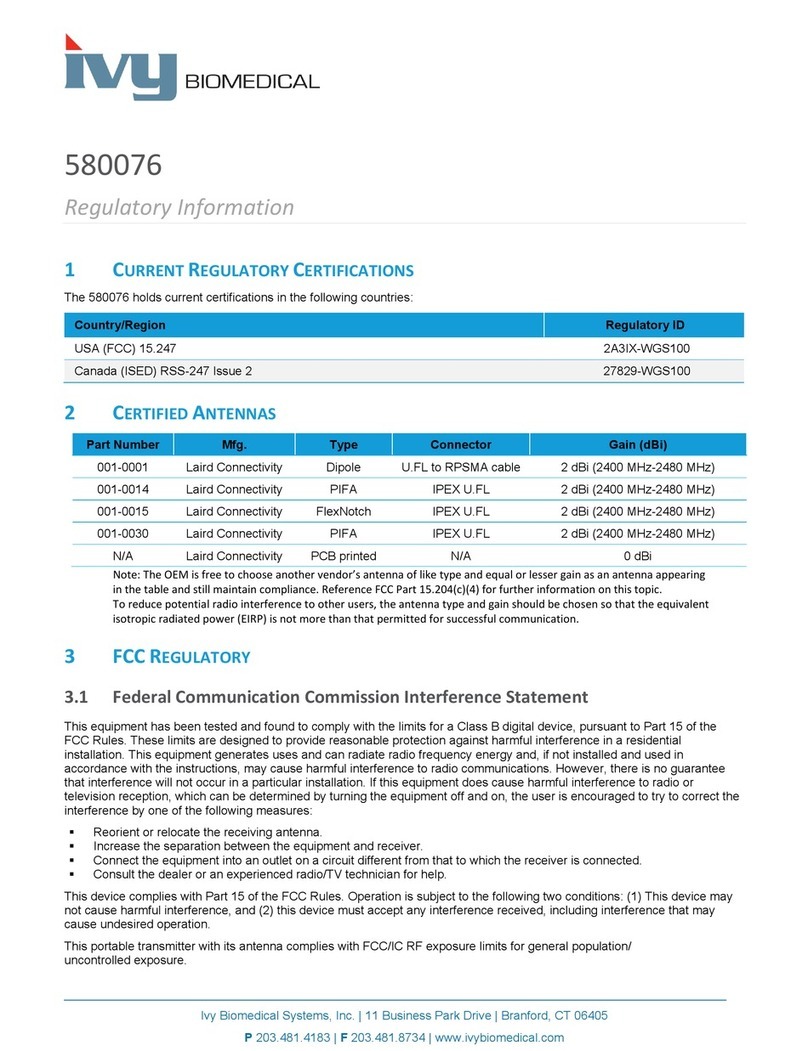
Table of Contents
Model 3150-B Operation Manual i
Table of Contents
WARRANTY ...................................................................................................................................................... iii
INTRODUCTION ................................................................................................................................................1
SAFETY ................................................................................................................................................................2
Electrical................................................................................................................................................................2
Explosion ...............................................................................................................................................................2
Patient Connections..............................................................................................................................................3
MRI........................................................................................................................................................................3
Pacemakers ...........................................................................................................................................................3
Electrosurgery Protection....................................................................................................................................3
Defibrillation Protection ......................................................................................................................................3
EMC.......................................................................................................................................................................3
Electromagnetic Compatibility IEC 60601-1-2:2001 ..............................................................................3
Description of Warning Labels ...........................................................................................................................7
MONITOR DESCRIPTION ...............................................................................................................................8
Classification.........................................................................................................................................................9
Control and Indicators.......................................................................................................................................10
Basic Keys..............................................................................................................................................10
Programmable Keys ...............................................................................................................................11
Menu Structure.......................................................................................................................................12
Display ...................................................................................................................................................13
Alarm Messages .....................................................................................................................................14
Rear Panel ..............................................................................................................................................14
Fuse Ratings ...........................................................................................................................................15
MONITOR SETUP ............................................................................................................................................16
Set up the instrument for operation..................................................................................................................16
Change Mains Voltage .......................................................................................................................................16
Set the Language ................................................................................................................................................16
Set Time, Date, and Audio .................................................................................................................................16
Trace Speed.........................................................................................................................................................17
Default Settings...................................................................................................................................................17
SYNCHRONIZED OUTPUT (TRIGGER) .....................................................................................................18
The Synch Pulse..................................................................................................................................................18
Trigger-Mark Display ........................................................................................................................................18
Polarity Lock (P-Lock).......................................................................................................................................18
ECG MONITORING.........................................................................................................................................19
Safety Considerations.........................................................................................................................................19
Patient Connections............................................................................................................................................20
ECG Electrodes ..................................................................................................................................................21
Impedance Measurement ..................................................................................................................................21
ECG Waveform Amplitude (Size).....................................................................................................................22
Lead Selection .....................................................................................................................................................23
Low Signal Message ...........................................................................................................................................24
ECG Notch Filter................................................................................................................................................24
Alarm Limits.......................................................................................................................................................25
Pacemaker...........................................................................................................................................................25