880.03 911 SHELLwww.kong.it
5
Original Italian version of 04/07/2018
Warning: not suitable for
use in ATEX environments
(Directive 94/9 / EC)
The information provided by the
manufacturer (hereafter the information)
must be read and understood by the
user before using the device. The
information comprises a description of the
characteristics, performance, assembly,
disassembly, maintenance, storage,
disinfection, etc. of the device, even if there
are also some suggested uses that do not
necessarily need to be considered in a user
manual designed for real situations.
WARNINGS AND
LIMITATIONS OF USE:
- This device may only be used by
persons who are physically fit, trained
(informed and trained) in its use and who
have specific experience regarding the
movement of patients or else, in training
activities, by persons who are under the
direct supervision of trainers / supervisors
ensuring their safety;
- Do not use the device until you have read
and understood this user manual in its
entirety;
- Comply scrupulously with the
manufacturer’s information: incorrect use
of the device is dangerous;
- It is absolutely forbidden to modify and /
or repair the device;
- Both before and after use, all the
checks described in chapter 7 must
be performed If the user has the
slightest doubt about the efficiency
of the device, he must replace it
immediately;
- Non-compliant use, warping, accidental
dropping, wear, chemical contamination,
2
GENERAL INFORMATION
exposure to temperatures below -30 °
C or higher than + 50 ° C in the case of
textile / plastic components / devices, and
+ 100 ° C in the case of metal components
/ devices, are just some examples of
causes that can reduce, limit and even
end the life of the device;
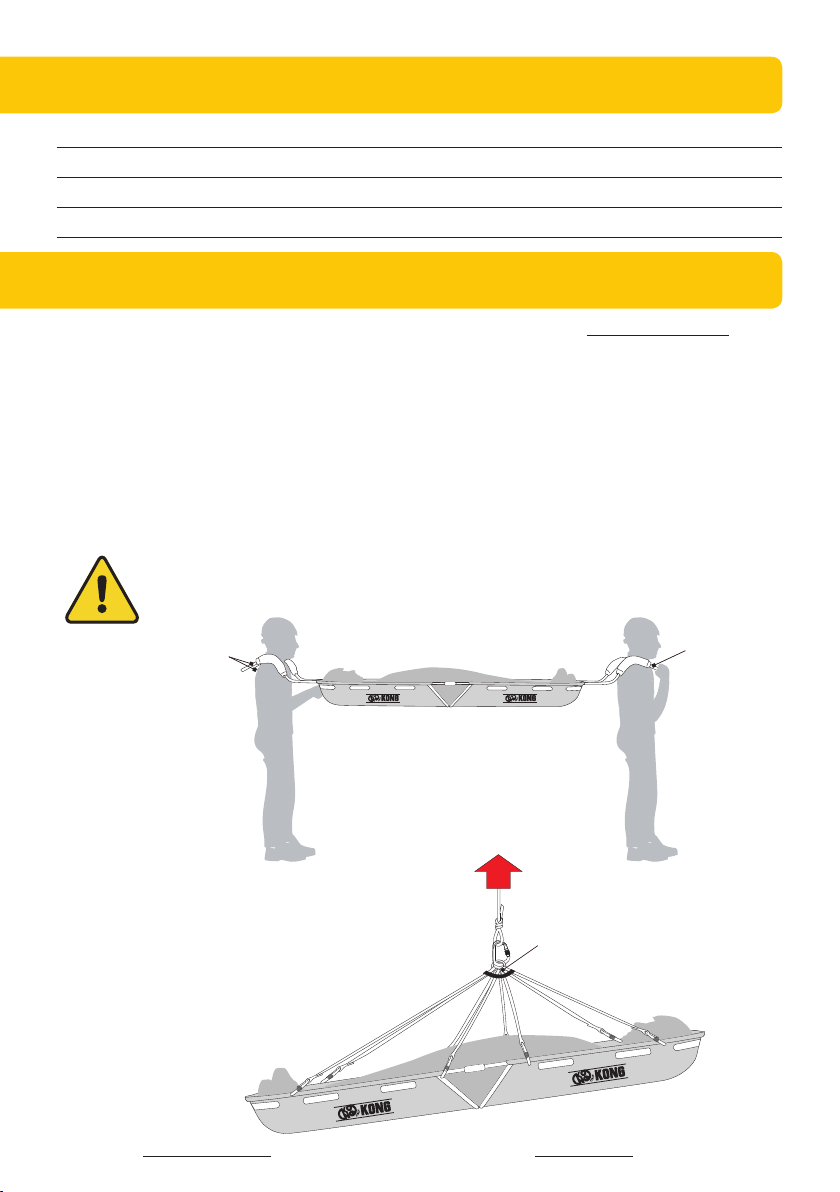
- Before any patient recovery operation,
make sure that the weight to be carried
does not exceed the capacity stated in
paragraph 3.3;
- To reduce the risk of exposure /
transmission of infectious diseases,
perform cleaning and disinfection of the
device as described in chapter 5;
- Incorrect use of the systems designed
to physically secure the patient could
compromise the patient’s safety;
- Always check the compatibility of any
devices used in combination with each
other against the relevant information
provided by the manufacturer;
- The use of spare parts or accessories
other than those listed in paragraph 3.4
can be dangerous;
- Avoid exposing the device to sources of
heat or contact with chemicals. Reduce
direct exposure to the sun, as necessary.
At low temperatures and in the presence
of moisture, ice formation may reduce
materials’ flexibility and increase the risk
of cutting and abrasion posed by textile
and synthetic devices.
All of our devices are tested / checked piece
by piece in accordance with the Quality
System procedures certified under the UNI
EN ISO 9001 standard. Laboratory tests,
inspections, information and standards are
not always able to reproduce actual practice,
so that the results obtained when the
device is used under real conditions in the
natural environment may differ, sometimes
significantly. The best information is always
obtained from continued practice with
using the device under the supervision of
competent / expert / qualified persons.
CHAPTER 2