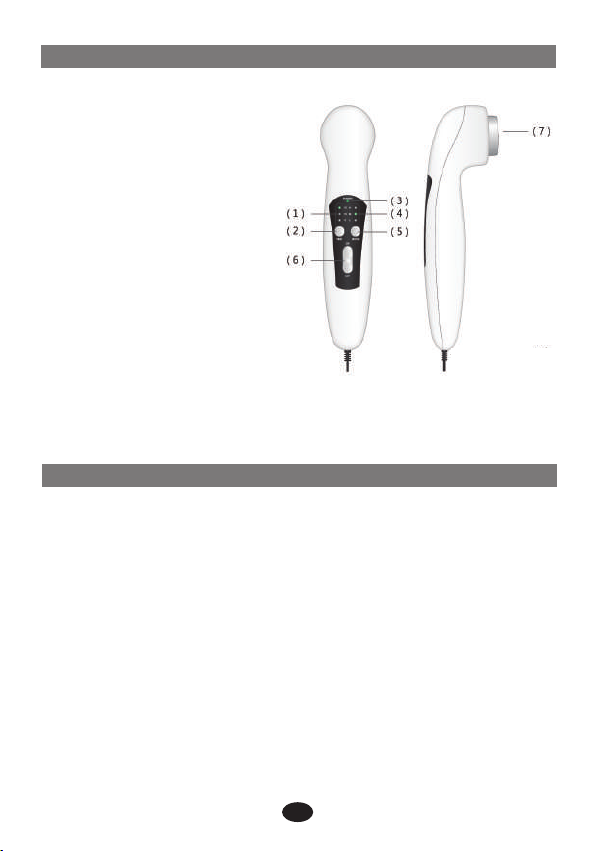
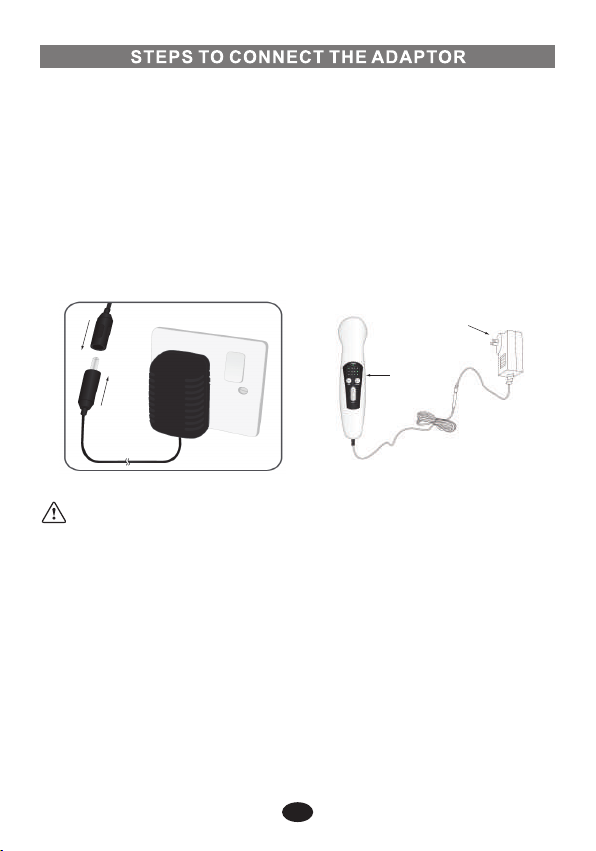
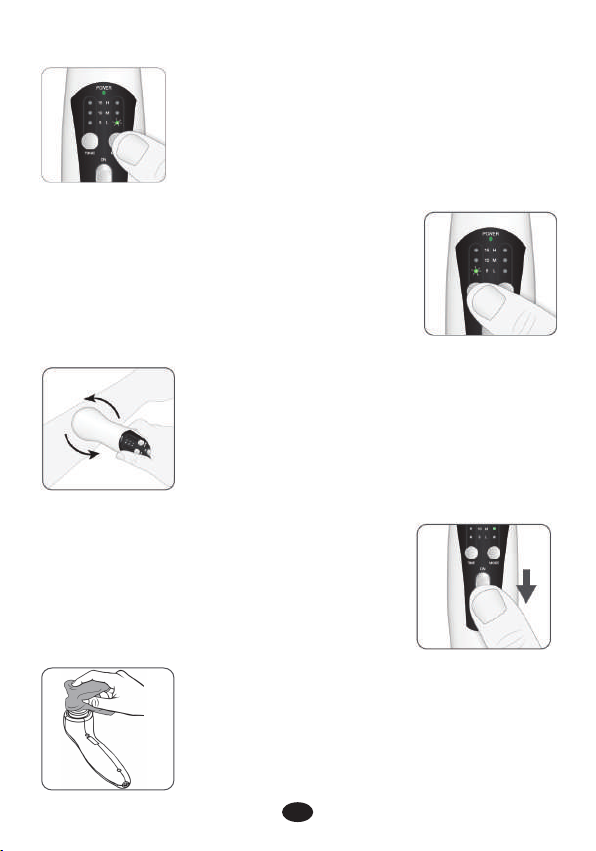
6
1. Always use this device under the directions of a physician.
2. Patients with the following diseases, symptoms or conditions should
not use the device:
● During pregnancy or menstrual cycle.
● Acute disease, heart disease, tubercle disease, facial neuralgia (sharp
facial pain), pernicious tumor, hemophilia, high fever, abnormal blood
pressure, or under any unhealthy conditions.
● On patients with sensitive physical conditions, ringworm, dermatitis,
and any infectious disease.
●
infants/small children, mentally disabled individuals, individuals under
● Product should not be applied on the following areas: any wounds, the
mouth, facial neuralgia (sharp painful) spots, surgical areas, sunburned
skin, sensitive skin and over skin implants made of metal, plastic or
silicone materials.
● Do not use with other electronic equipment, such as ECG machine etc.,
even if this device conforms to the EMC requirements.
3. DO NOT use on the thoracic region if you have a pacemaker.
4. DO NOT use on areas where malignant tumors are present.
5. DO NOT use on the areas of blood inhibited tissue, because there is not
enough blood supplied to the area to meet the metabolic demand, and
this could result in tissue necrosis (tissue death).
6. DO NOT use the device on persons with bleeding issues/disorders.
7. DO NOT use on areas under anesthesia.
WARNING
●The device complies completely with all parts of 21 CFR 1050.10
under the performance standard for sonic, infrasonic and ultrasonic
radiation‐emitting products.
●Use of controls or adjustments to performance of procedures other
ultrasonic energy.
CAUTIONS